- Joined
- Jun 6, 2012
- Messages
- 153
- Reaction score
- 0
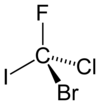
Isn't there some trick for chirality problems that you don't need to reorient the molecule? Like if you just skip over the lowest priority substituent when following the circle (CCW or CW) don't you just assign the opposite value to what that method would yield? Like if you skip over a hydrogen when following the order of substituents on the carbon and you get an R configuration, you just assign it S instead?
Also, if I have two carbons attached to the chiral center, and one of those carbons is a carbonyl, but the other carbon has a hydroxyl group, I assign the carbonyl the higher priority because it is as if it is attached to two oxygens, right?
If someone could please provide some clarification it would be very much appreciated!
🙂
Also, if I have two carbons attached to the chiral center, and one of those carbons is a carbonyl, but the other carbon has a hydroxyl group, I assign the carbonyl the higher priority because it is as if it is attached to two oxygens, right?
If someone could please provide some clarification it would be very much appreciated!
🙂