D
deleted647690
According to this picture from uworld, the OR portion of an ester is considered electron withdrawing, and the nitrogen portion of an amide is considered electron donating. I suppose I can see how, in the amide, the carbonyl oxygen is more electronegative than the amide nitrogen, thus it pulls electron density, making the Nitrogen donating.
However, why is an ester considered electron withdrawing? Both the Nitrogen and the oxygen of amides and esters, respectively, have lone pairs of electrons that they could share towards the carbonyl group. Wouldn't this make them both electron donating?
Carboxylic acid OH group is considered electron withdrawing. Ester OR group is considered electron withdrawing. Amide NRH group is considered electron donating. Is this because N is less electronegative than the carbonyl oxygen? All of these groups have lone pairs to donate and contribute to resonance stability, so I'd think they could all be electron donating. At the same time, they are all highly electronegative atoms, so could be seen as electron withdrawing as well.
Alternatively, why is an alkoxy group (-OR) considered electron donating? Again, the oxygen here has electron lone pairs to donate. At the same time, Oxygen is highly electronegative, so I'd expect it to have some withdrawing capacity.
I'm confused by this, as I see an oxygen atom and think that it would be electron withdrawing due to high electronegativity. This doesn't seem to be the case, as seen with alkoxy groups.
In addition, in the case of an ester, both oxygen atoms would be competing in terms of electronegativity difference.
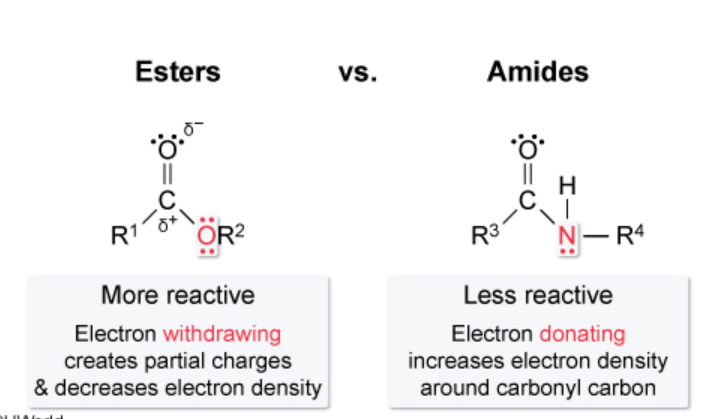
However, why is an ester considered electron withdrawing? Both the Nitrogen and the oxygen of amides and esters, respectively, have lone pairs of electrons that they could share towards the carbonyl group. Wouldn't this make them both electron donating?
Carboxylic acid OH group is considered electron withdrawing. Ester OR group is considered electron withdrawing. Amide NRH group is considered electron donating. Is this because N is less electronegative than the carbonyl oxygen? All of these groups have lone pairs to donate and contribute to resonance stability, so I'd think they could all be electron donating. At the same time, they are all highly electronegative atoms, so could be seen as electron withdrawing as well.
Alternatively, why is an alkoxy group (-OR) considered electron donating? Again, the oxygen here has electron lone pairs to donate. At the same time, Oxygen is highly electronegative, so I'd expect it to have some withdrawing capacity.
I'm confused by this, as I see an oxygen atom and think that it would be electron withdrawing due to high electronegativity. This doesn't seem to be the case, as seen with alkoxy groups.
In addition, in the case of an ester, both oxygen atoms would be competing in terms of electronegativity difference.
Last edited by a moderator:
