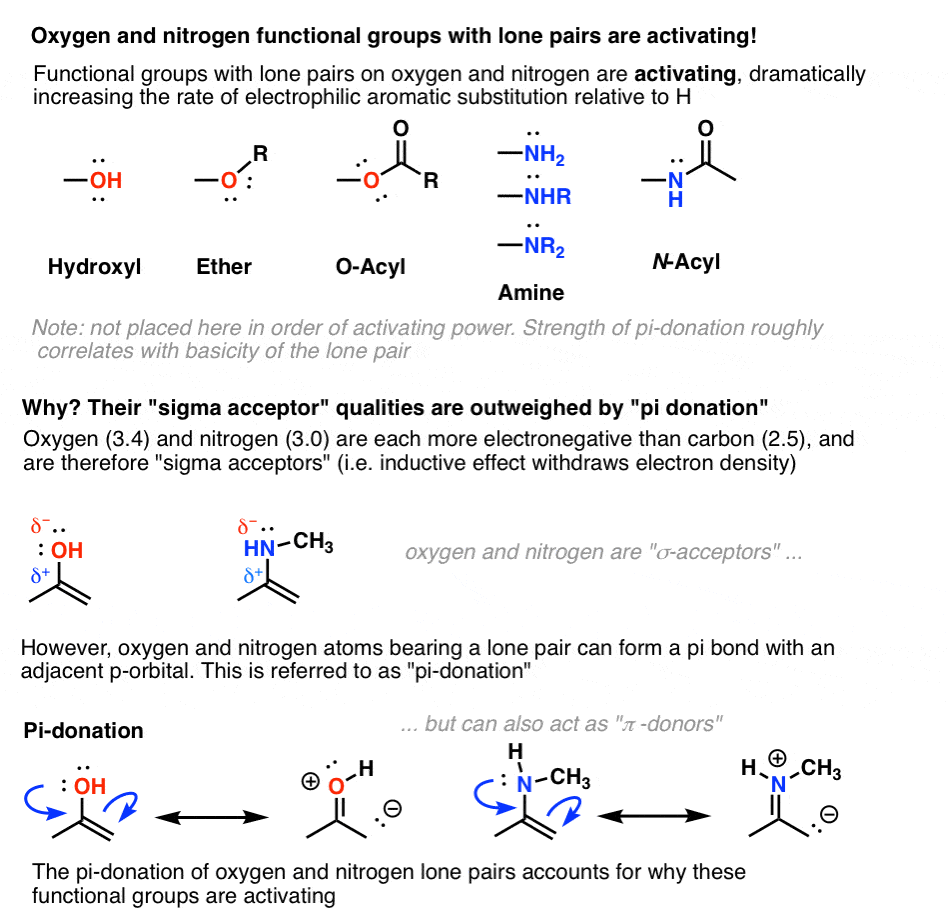
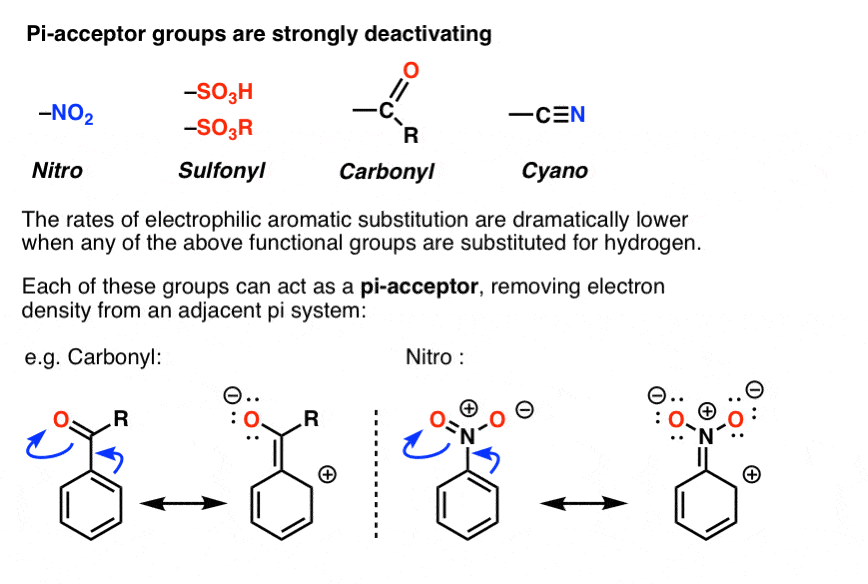
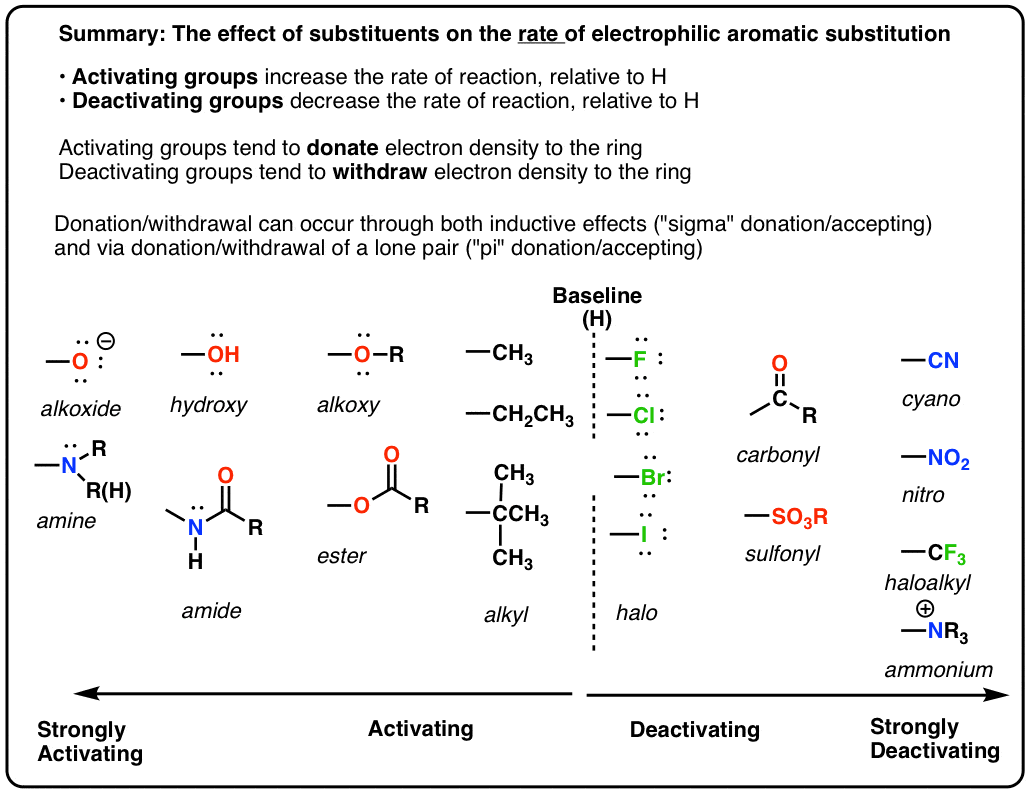
Hi,
I am unsure how you would determine if a group is electron withdrawing or electron donating by the resonance forms.
My confusion stems from the amide group...I thought from the resonance that the carbonyl group would be electron withdrawing (by getting a partial negative charge) and nitrogen would be electron donating (from its lone pair). But TBR mentioned that the oxygen would actually be electron donating and the most basic site.
If carbonyl in an amide is electron donating by resonance, how is carbonyl in a carboxylic acid electron withdrawing by resonance?
(Also: by inductive effects, the oxygen is more electronegative so would be electron withdrawing, but resonance takes priority over induction...right?)
Sorry this may sound like a dumb question but I realize that I haven't actually understood the foundation of this concept and I can't seem to find a straightforward answer anywhere.
Thanks in advance if someone could clear this confusion...been at this all day
I am unsure how you would determine if a group is electron withdrawing or electron donating by the resonance forms.
My confusion stems from the amide group...I thought from the resonance that the carbonyl group would be electron withdrawing (by getting a partial negative charge) and nitrogen would be electron donating (from its lone pair). But TBR mentioned that the oxygen would actually be electron donating and the most basic site.
If carbonyl in an amide is electron donating by resonance, how is carbonyl in a carboxylic acid electron withdrawing by resonance?
(Also: by inductive effects, the oxygen is more electronegative so would be electron withdrawing, but resonance takes priority over induction...right?)
Sorry this may sound like a dumb question but I realize that I haven't actually understood the foundation of this concept and I can't seem to find a straightforward answer anywhere.
Thanks in advance if someone could clear this confusion...been at this all day