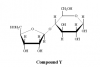
Is the sugar on the left of this disacharide d or l, and why?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
D or L Sugar?
- Thread starter joshto
- Start date
- Joined
- Oct 20, 2009
- Messages
- 2,115
- Reaction score
- 498
It's a D sugar. The convention is to draw CH2OH pointing up on D sugars; if you were to flip it, then it'd be an L sugar.
Is the sugar on the left of this disacharide d or l, and why?
- Joined
- Oct 20, 2009
- Messages
- 2,115
- Reaction score
- 498
I think you are confusing D and L with R and S. Remember in D and L you are relating the molecule to the structure of glyceraldehyde. I think wikipedia might help you here: http://en.wikipedia.org/wiki/Chirality_(chemistry).
What about in terms of actually figuring out chirality on C#5?
How do you determine C5's chirality from this sugar's ring structure?
- Joined
- Dec 11, 2009
- Messages
- 181
- Reaction score
- 1
Hmm so from my point of view and my logic. I know this is a Haworth projection and you know from TBR that in Haworth projections, if it is pointing up, it is associated with the left side of the straight chain of the carbohydrate. If it is on the right side of the straight chain, it should be pointing down. Thus from this, you can now form the straight chain from the Haworth projection. Thus, you would look at the carbon before the last carbon and check if it is on the right of the left side of the straight chain. If it is located on the right side, it would be D, if it is on the left side, it would be L. Then look at the groups to determine R/S. Again, I'm not pro and I don't know if there's some super shortcut but I would do that.
Hopefully that helps
Hopefully that helps
- Joined
- Oct 20, 2009
- Messages
- 2,115
- Reaction score
- 498
Yup, this is accurate. The stereochemistry can be different at each chiral center, so watch out for that.
Hmm so from my point of view and my logic. I know this is a Haworth projection and you know from TBR that in Haworth projections, if it is pointing up, it is associated with the left side of the straight chain of the carbohydrate. If it is on the right side of the straight chain, it should be pointing down. Thus from this, you can now form the straight chain from the Haworth projection. Thus, you would look at the carbon before the last carbon and check if it is on the right of the left side of the straight chain. If it is located on the right side, it would be D, if it is on the left side, it would be L. Then look at the groups to determine R/S. Again, I'm not pro and I don't know if there's some super shortcut but I would do that.
Hopefully that helps
Hmm so from my point of view and my logic. I know this is a Haworth projection and you know from TBR that in Haworth projections, if it is pointing up, it is associated with the left side of the straight chain of the carbohydrate. If it is on the right side of the straight chain, it should be pointing down. Thus from this, you can now form the straight chain from the Haworth projection. Thus, you would look at the carbon before the last carbon and check if it is on the right of the left side of the straight chain. If it is located on the right side, it would be D, if it is on the left side, it would be L. Then look at the groups to determine R/S. Again, I'm not pro and I don't know if there's some super shortcut but I would do that.
Hopefully that helps
i used this method as well. the problem is that, with this method, the sugar turns out to be an L sugar, which is not true.
I think you can't really use what's pointing up or what's pointing down on the penultimate chiral carbon to derive the Fisher projection. I think you have to see that if the "CH2OH" that's bonded to the penultimate chiral carbon is pointing up, then it's a D.
Also, R and S don't relate to D and L. I think the sugar on the left side is a five-carbon sugar, so carbon #5 is ACHIRAL (which is CH2OH), since it's bonded to 2 H's.
Also, R and S don't relate to D and L. I think the sugar on the left side is a five-carbon sugar, so carbon #5 is ACHIRAL (which is CH2OH), since it's bonded to 2 H's.
Similar threads
- Replies
- 1
- Views
- 687