- Joined
- May 3, 2015
- Messages
- 171
- Reaction score
- 49
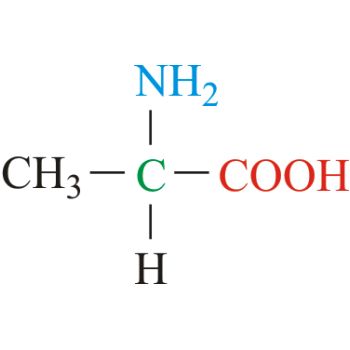
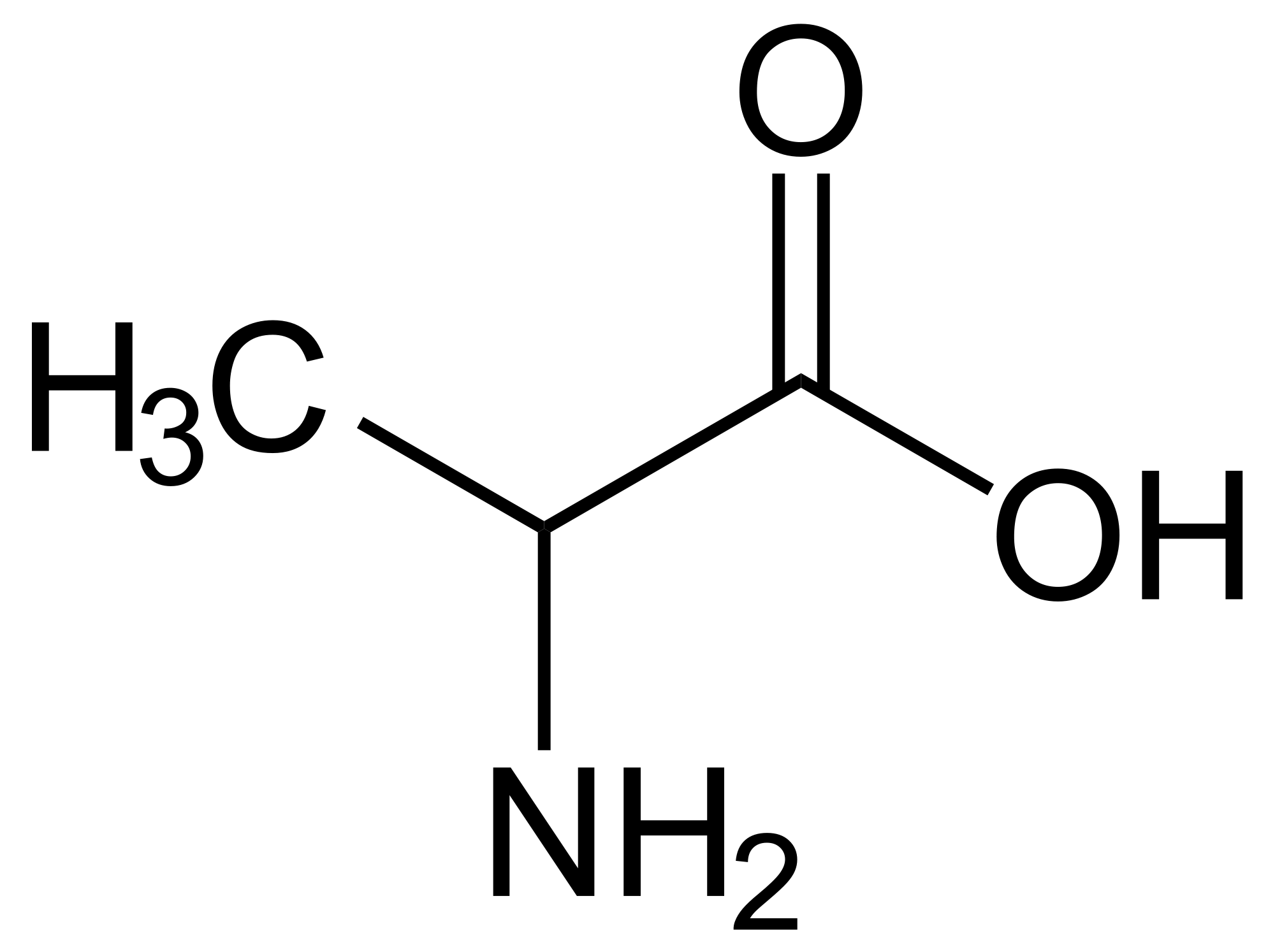
I'm not really comfortable with amino acids, not at an intimate level at least. I know their structures inside and outside. But amino acids suffer bipolar disorder, sometimes being polar to even non-polar. But I know amino acids can be positive sometimes and flush out those negative feelings.
On a more serious note: how well do we need to know amino acids? Down to the every pKa of each substituent and the pIs?? How useful is that (especially those who have written the MCAT already)??
On a more serious note: how well do we need to know amino acids? Down to the every pKa of each substituent and the pIs?? How useful is that (especially those who have written the MCAT already)??