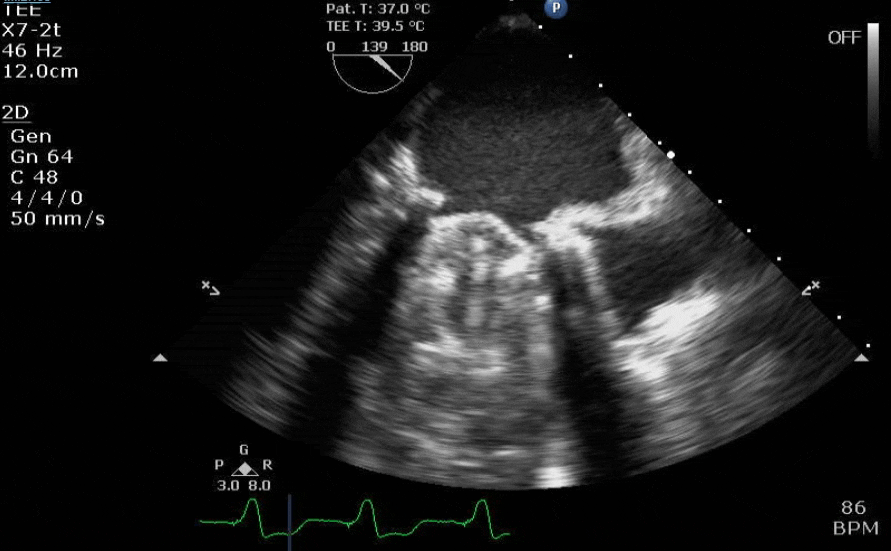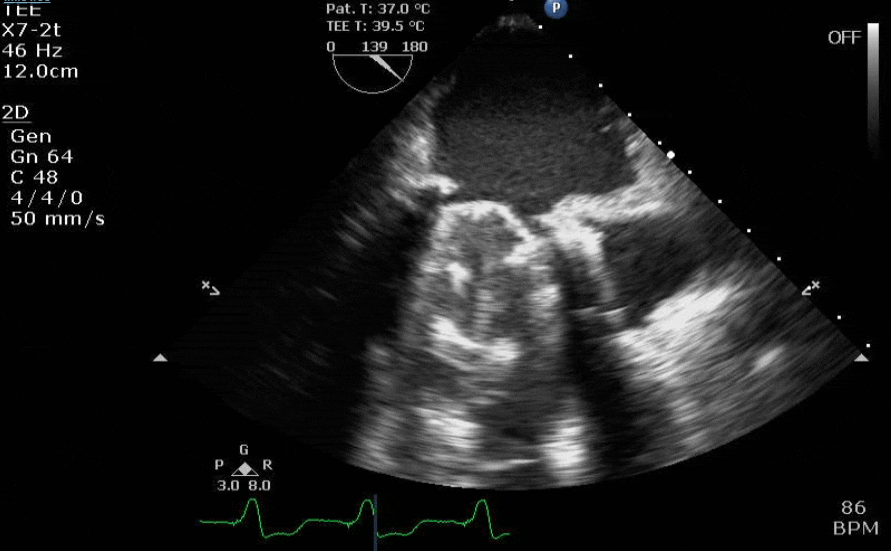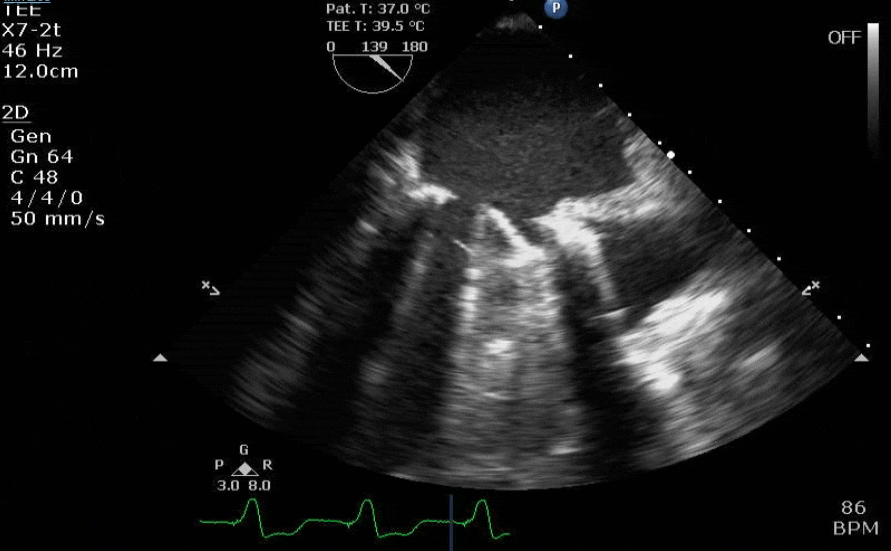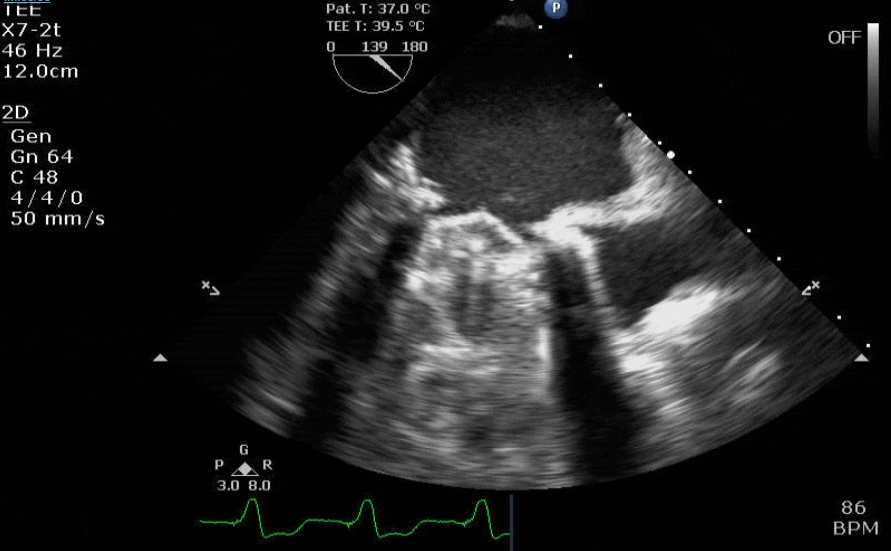
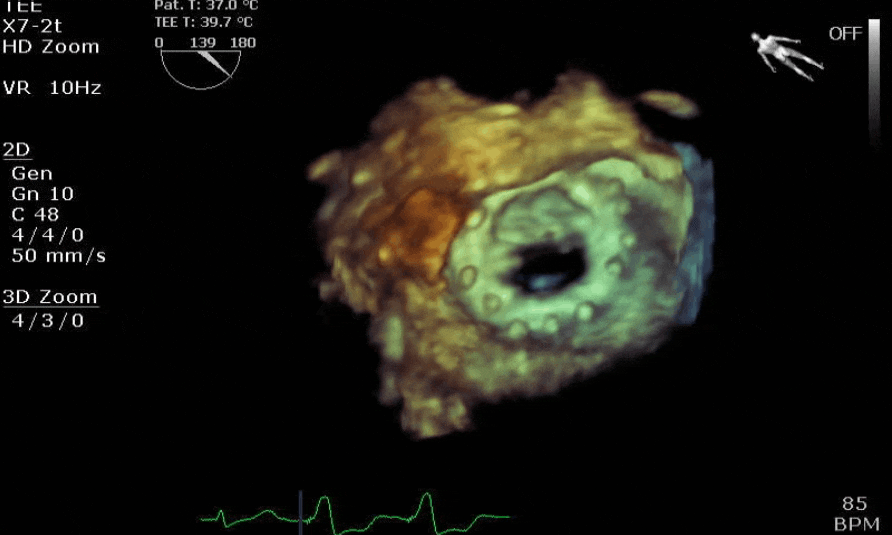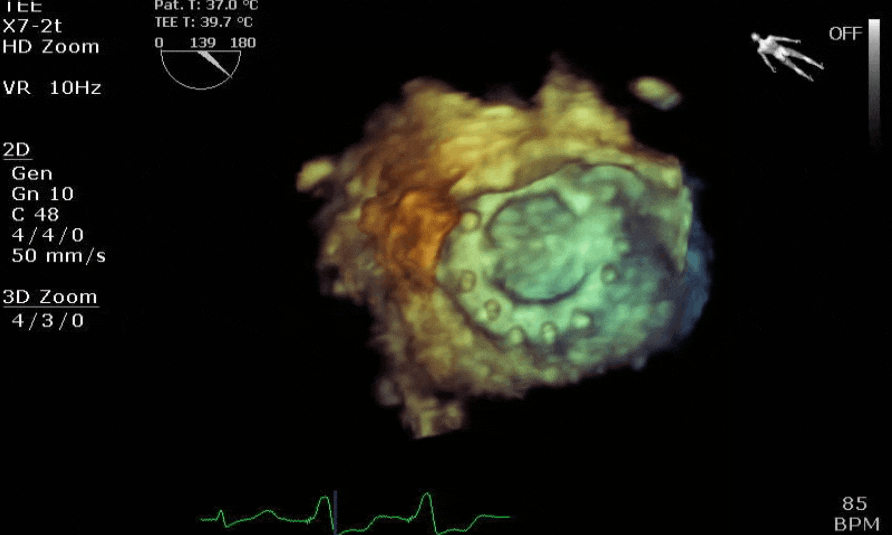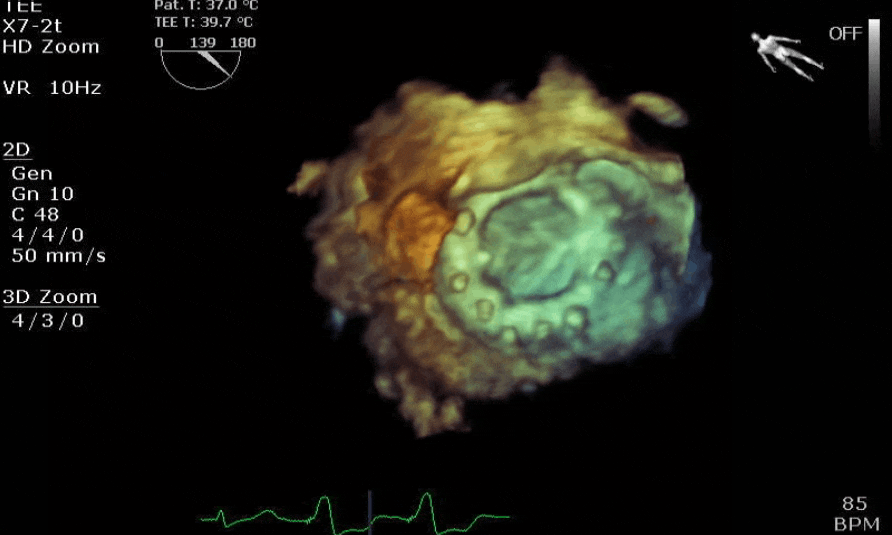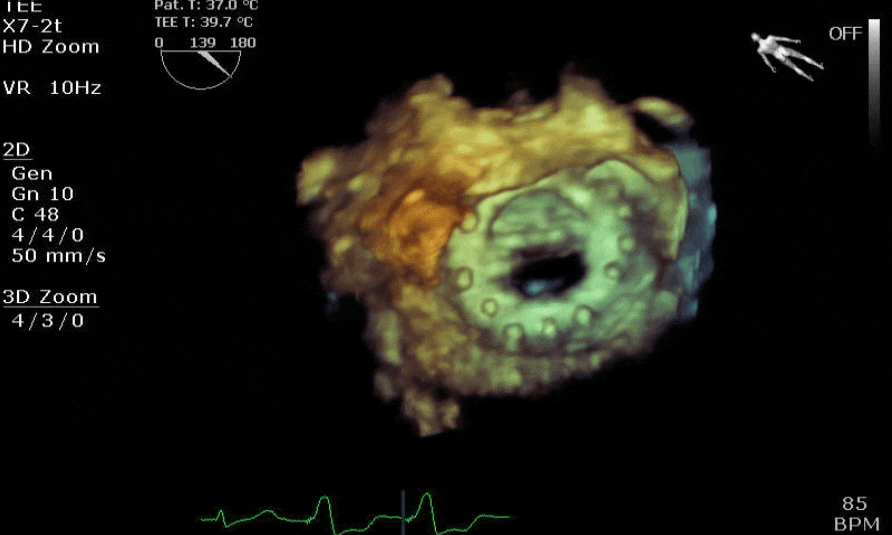
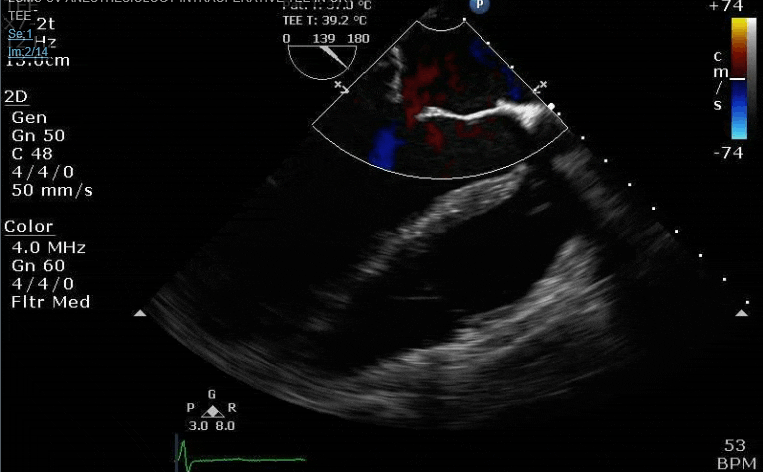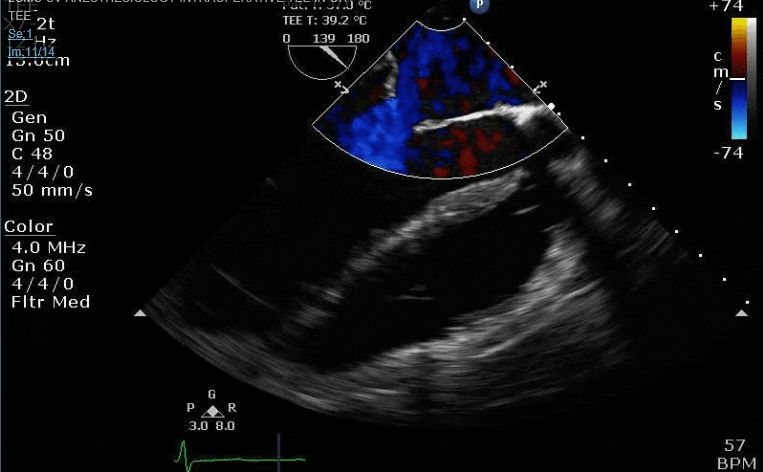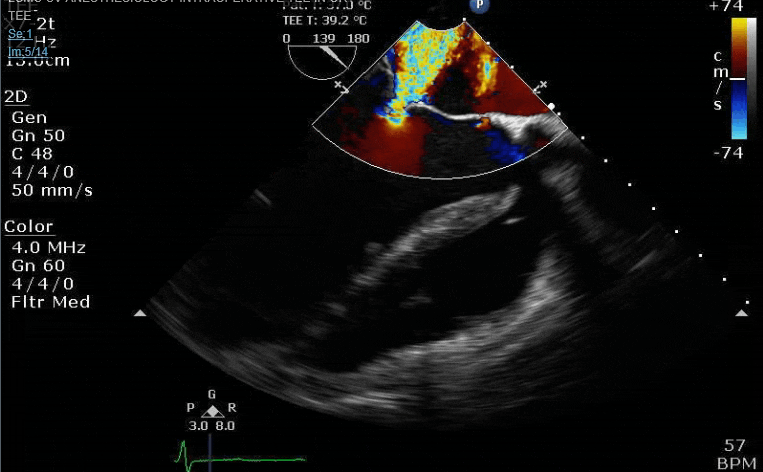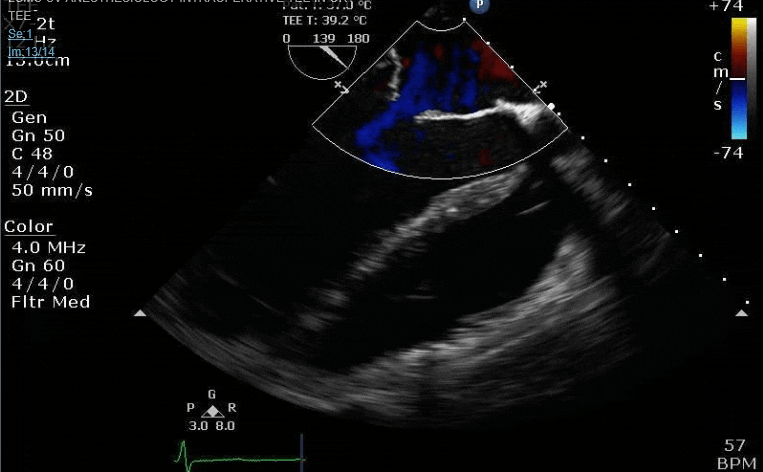
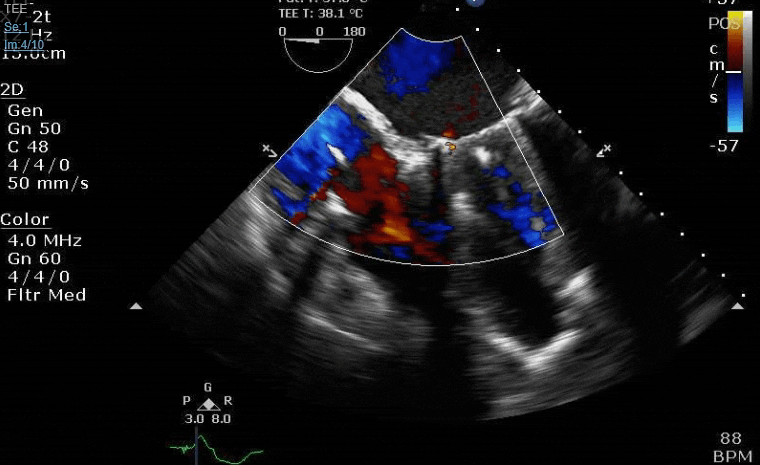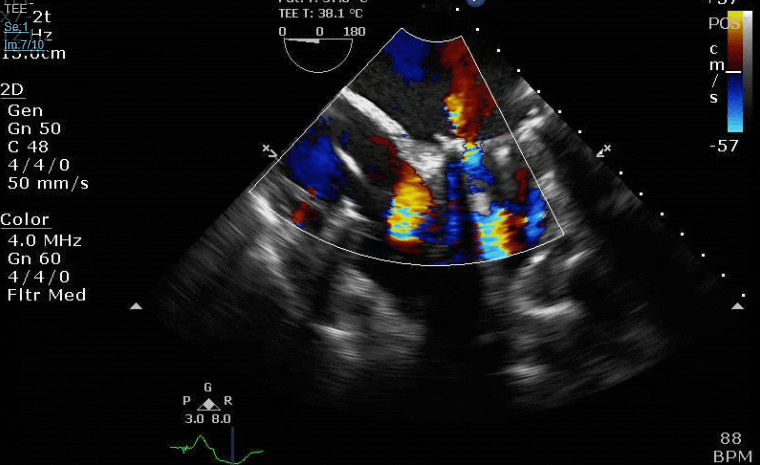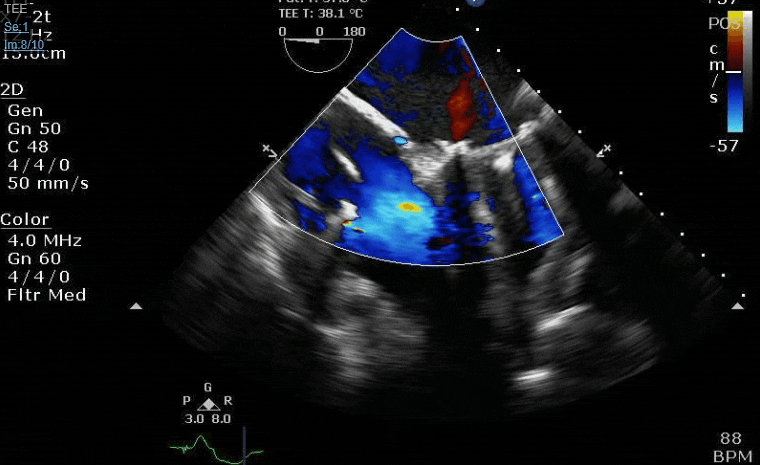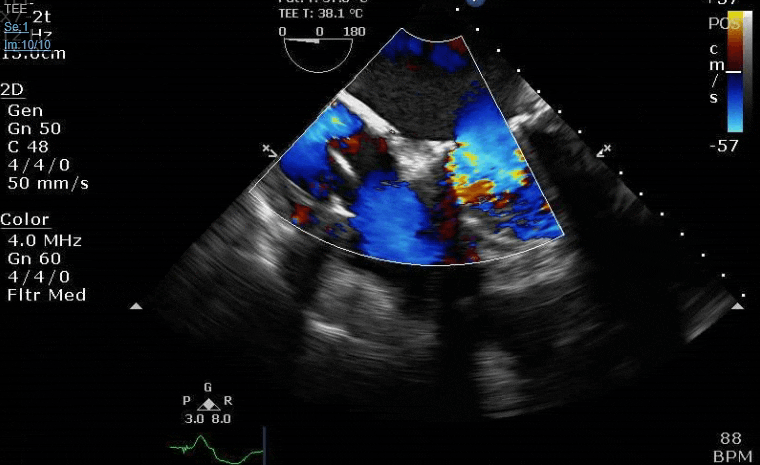
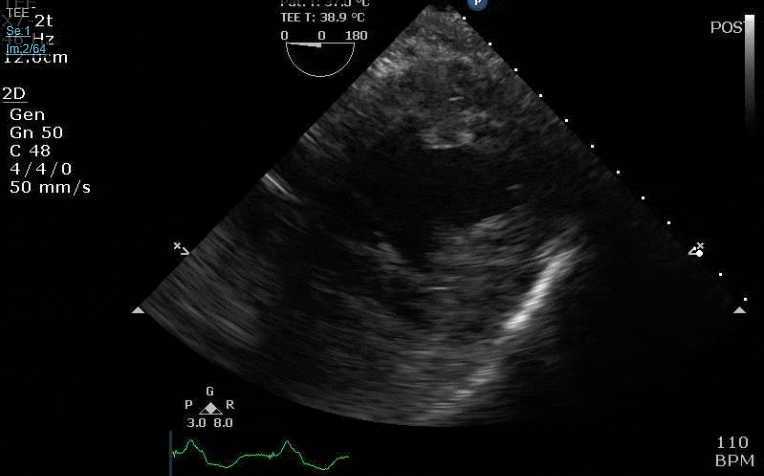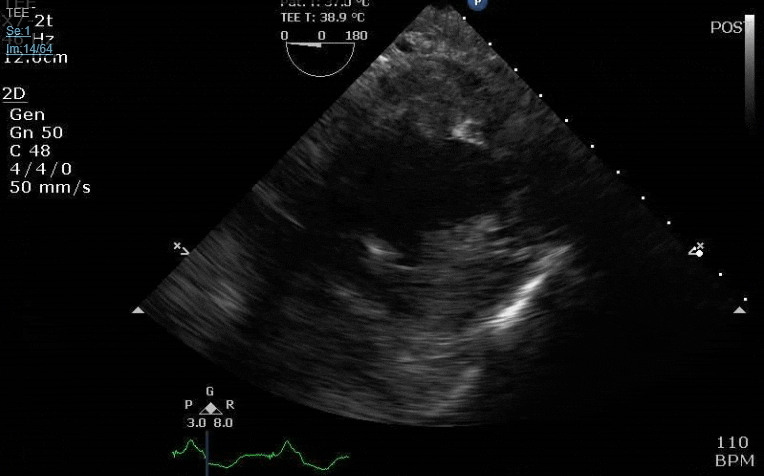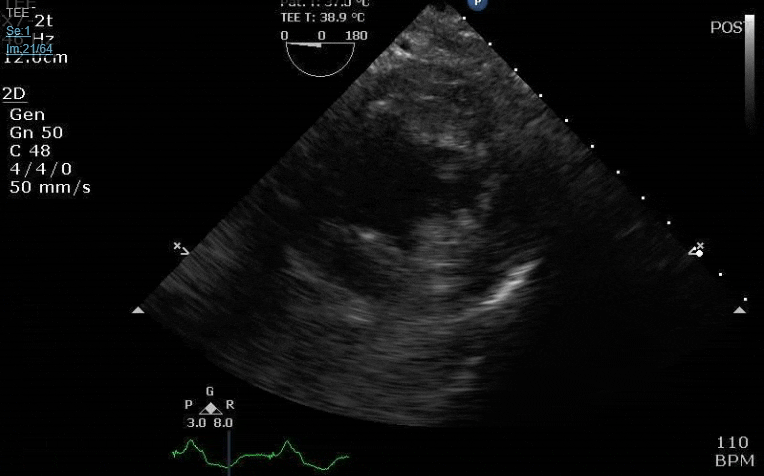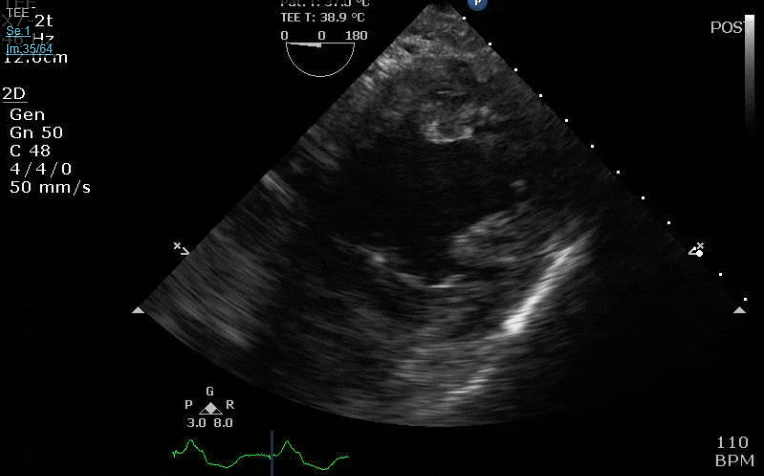
Lots happening here!
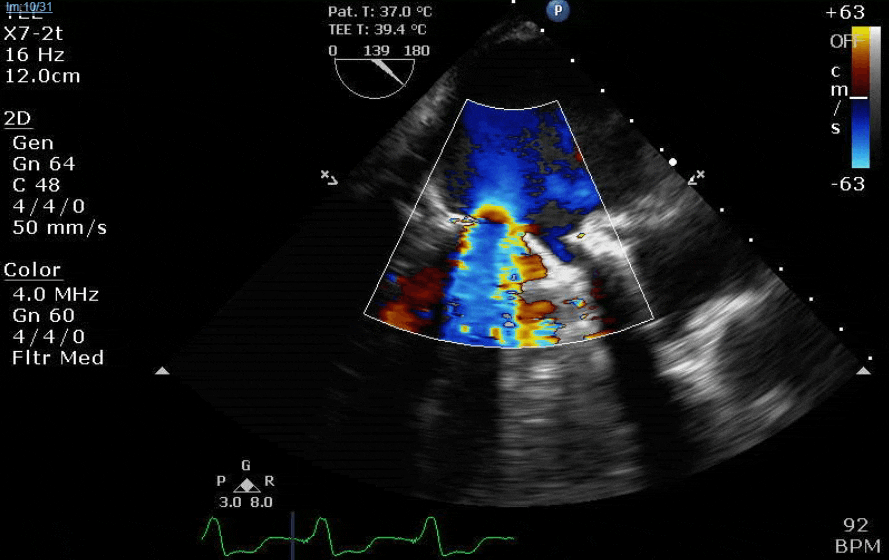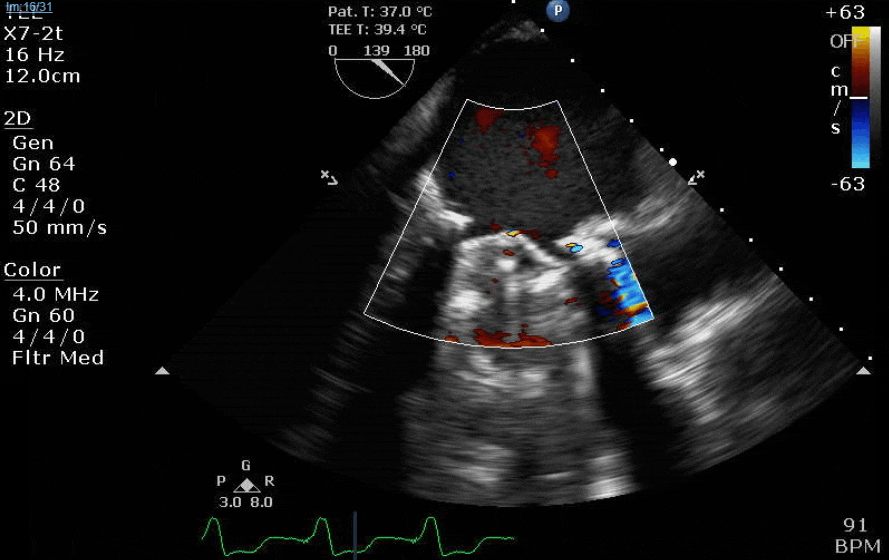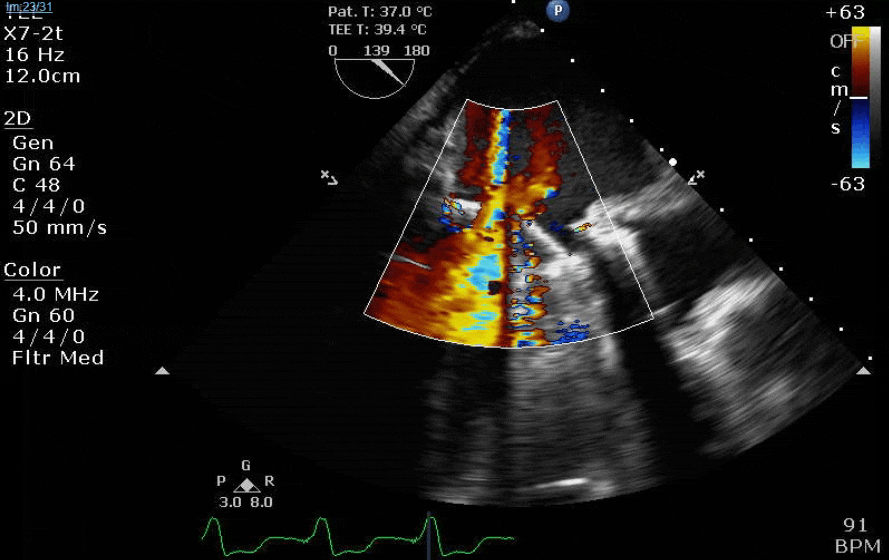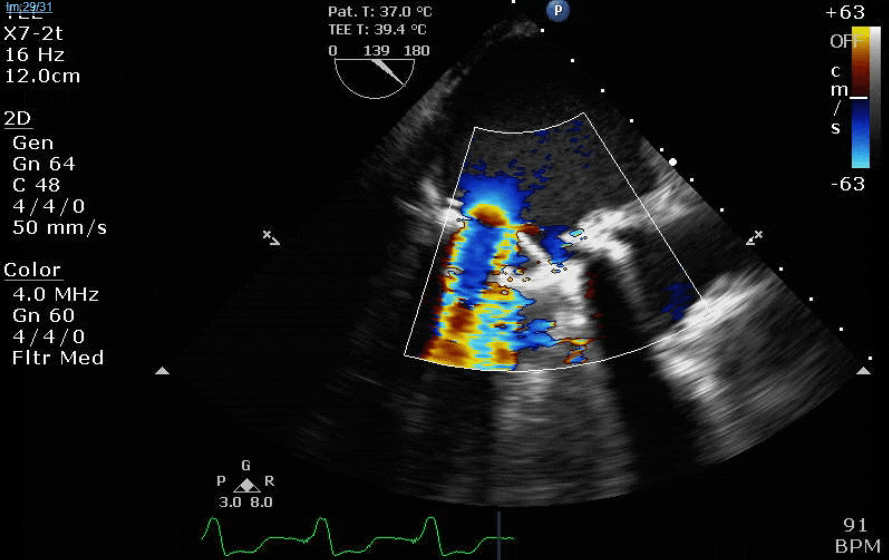
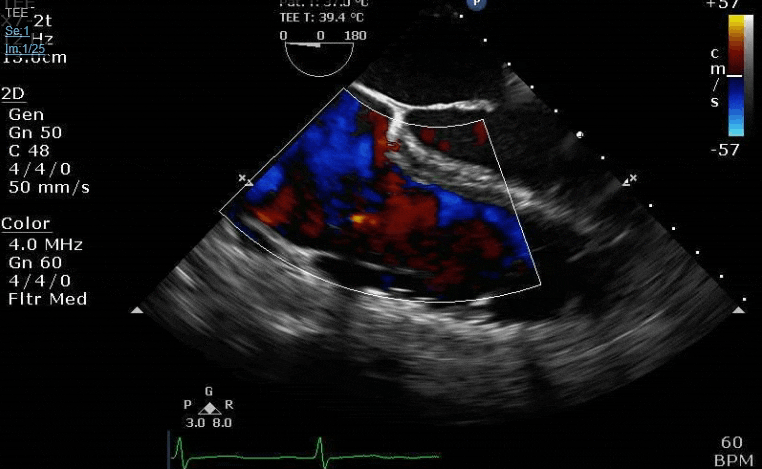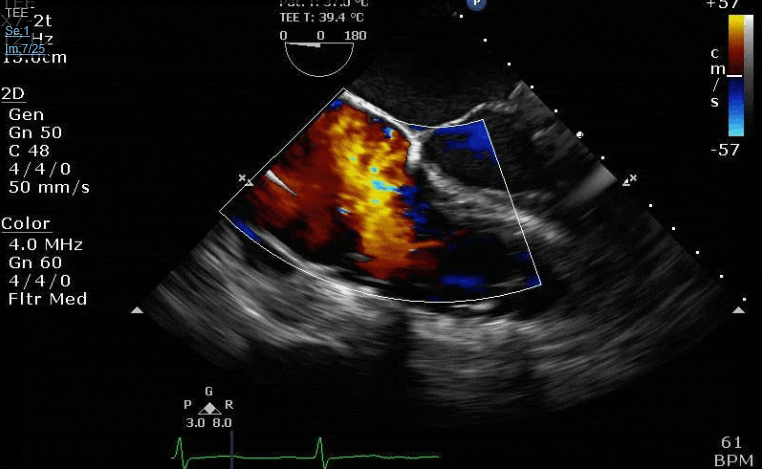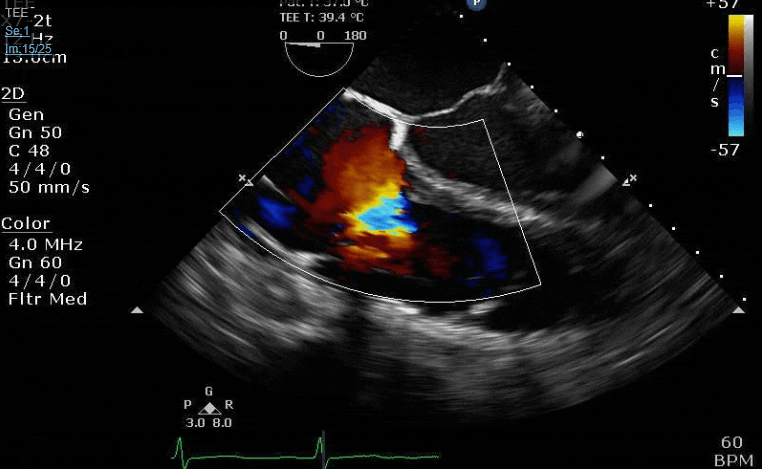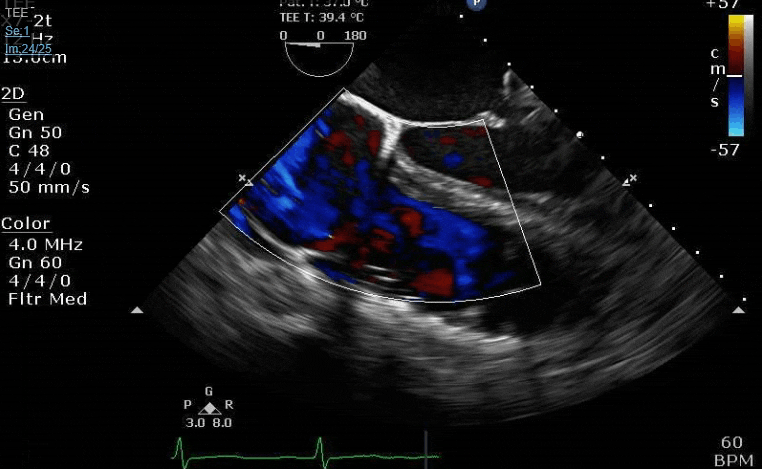
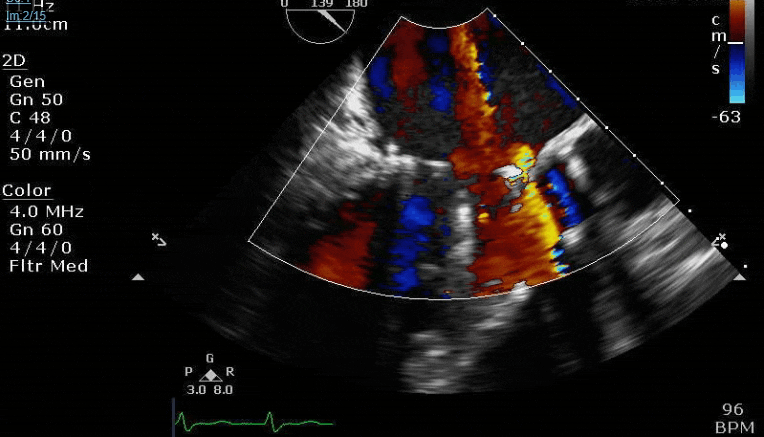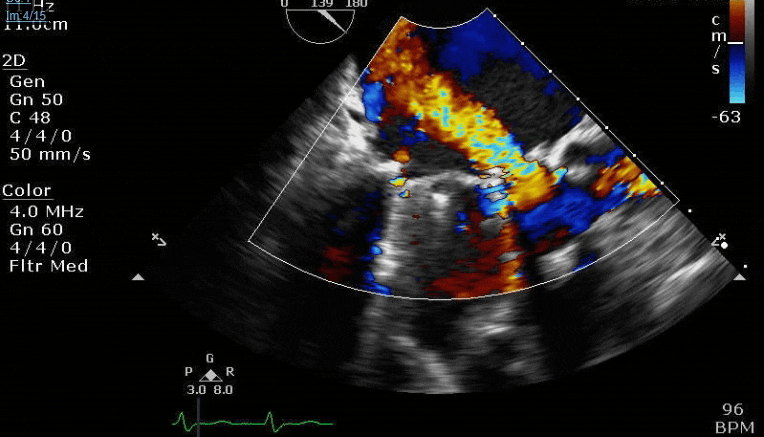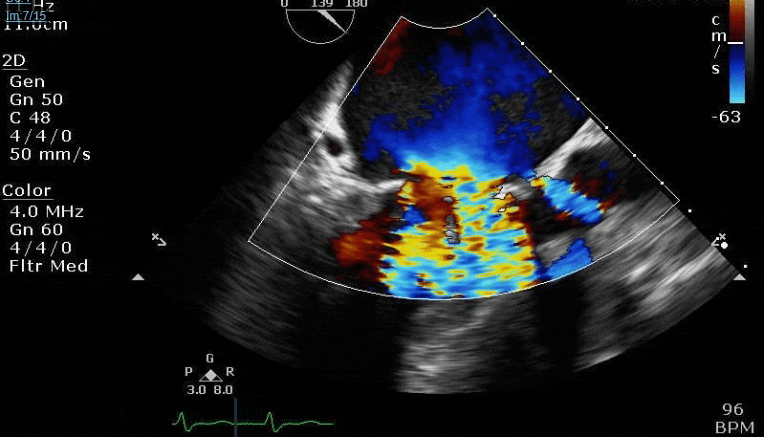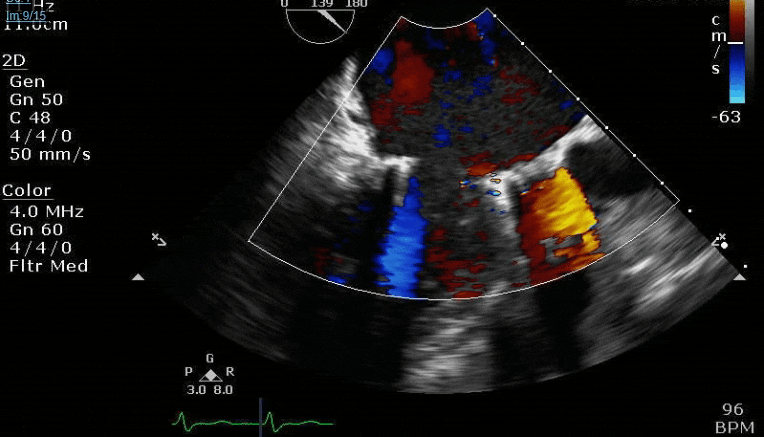
Looks like a perforation/hole from the LVOT to the LA that was made worse after pulling the MVR (I'd guess it's just from tissue distortion and tension from sutures).
Severe TR. And I feel like there's also a VSD, but my eyes may be deceiving me.
Was this done by a heart surgeon, or someone from EVS who stayed at a holiday inn express? Yikes.
Yikes… Was this the result of a (very) misguided attempt at chordal-sparing MVR, or what?
Yep, lot to unpack here. But first, some clinical history and context ( because of course it's easier to judge the result when all you have to go by are the clips).
Pt is a 47yo F. She's 360 lbs. This is her 3rd sternotomy with the prior two being for LA myxoma excisions. She is booked for an MVR/TVr/LAA clip/MAZE. She has a pre-op CMR with her LVEF being listed as 36% and her RVEF at 29%. This is obviously severe biventricular dysfunction given her regurgitant lesions.
Of course anyone in their right mind would get wires into the groin before sternotomy on this pt. Lead surgeon here gets frustrated though because her femoral vessels are buried under a million miles of fat and they can't access them even with ultrasound. Instead of doing a cutdown (or calling for fluoro and help from IC), he proceeds to sternotomy with the oscillating saw (of note, in 5 years I've never run into a [major] problem with this surgeon during redo sternotomies).
And as you can guess, this time, even with being really careful near the posterior table of the sternum, he saws right through the main PA and probably through some of the innominate vein. She immediately starts pouring blood and we start transfusing some prbc and she stabilizes. Lead surgeon is able to get enough exposure so that the surgery resident can hold manual pressure. Lead surgeon and 2nd surgeon then start working on groin dissection. Which takes FOREVER because they're super deep and not where they initially made incision. At this point we've been asleep for 2 hrs, but finally fems are cannulated and we go on bypass.
But having to do difficult redo dissections
on pump is the worst. Just the worst. The patient is getting all the downsides of cardiopulmonary bypass without the surgeon actually making substantive progress on repairing the lesions for which they came to surgery. She's amazingly fat and the working space in her mediastinum is tiny, so stopping the bleeding and doing enough dissection just to be able to centrally cannulate takes
another 2 hrs on pump. And she's so adhesed that doing the MAZE and clip is noted to be impossible anyway.
So 4 hrs into the case, we're clamped and pleged and lead surgeon can finally begin the actual valve replacement.
Of course we discover that in addition to being surrounded by fat and deep in the chest, the adhesions have rotated her heart so that the "surgeon's view" of the mitral through a transseptal approach is dog****. Surgeon cannot really visualize the intervalvular fibrosa that well even though we know there is a perforation in that region (but if he could see it, it does raise questions of how to spare the chords, leaflets or how to do a transposition technique, etc. Leaflet/chord sparing is important, doubly so when LV function is down). He does the best he can and gets the MVR in. TVr goes fine as well.
Clamp off, we start pacing and deair, fill up a bit and at this point you see the big old anterior PVL where that prior perforation was. And the TR is still high mild to moderate because the septal leaflet is probably still tethered. In regard to the mitral, you know the replacement is not adequate, but the flip side is, at this point,
the clamp time has been almost 2 hrs and the bypass time is over 4 hrs in a pt with severe biventricular dysfunction. Furthermore, the ICs who typically do impellas or help with VA ecmo (both of which we do pretty infrequently) are unavailable at the moment. Further furthermore, she is just profusely bleeding from everywhere. So even with MCS, unless you're gonna do it heparin free she is gonna bleed to death. Lead surgeon says the PVL is what it is and that there's no way he's clamping and pleging again. We manage to limp off on some high dose epi, milrinone, nitric, levo, vaso. Vac the chest, start a mini-MTP, she makes it to the unit.
Pt had her ups and downs (bedside washout for hemorrhage POD 0), but we managed to close the chest POD 3, extubate POD 9, weaned off all inotropes POD 10, and she managed to escape with no other organ failure. Future hemolysis or reop MVR notwithstanding, I'm still calling it a win.