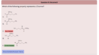
Can someone please explain why the answer is E and not C?
In C the relative position of the two highest priorities (T-Butyl and Br) are on the same side of the db, so I would expect Z? Unless I am overlooked something very obvious
In C the relative position of the two highest priorities (T-Butyl and Br) are on the same side of the db, so I would expect Z? Unless I am overlooked something very obvious