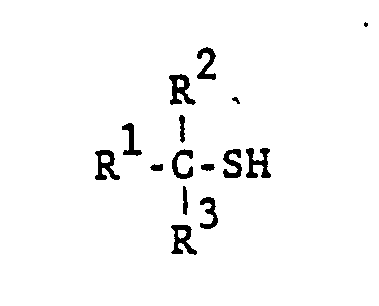D
deleted647690
There was a question I came across in my TBR orgo book
"Which of the following types of compounds is the MOST basic?"
A. Primary alcohols
B. Esters
C. Secondary amines
D. Tertiary thiols
It says that the answer is C because N is always more basic than the S in thiol. However, I thought N was more electronegative than S, and therefore more acidic, and less basic?
"Which of the following types of compounds is the MOST basic?"
A. Primary alcohols
B. Esters
C. Secondary amines
D. Tertiary thiols
It says that the answer is C because N is always more basic than the S in thiol. However, I thought N was more electronegative than S, and therefore more acidic, and less basic?