- Joined
- Dec 2, 2015
- Messages
- 438
- Reaction score
- 23
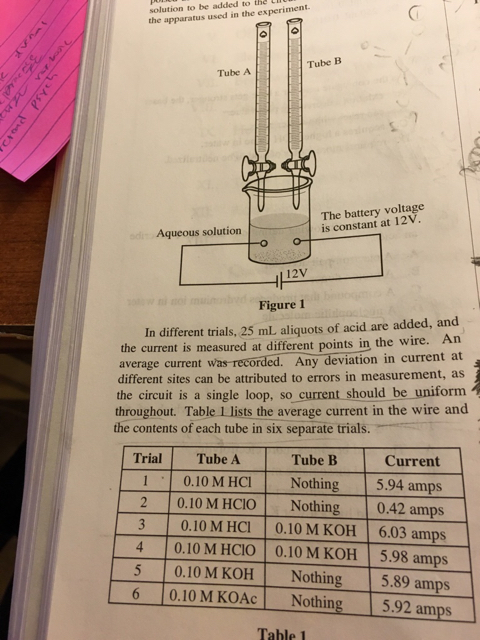
Confused about this question. I thought strong base and acid always create more ions in solution? Answer is d


Sent from my iPhone using SDN mobile
Sent from my iPhone using SDN mobile
