- Joined
- Sep 5, 2016
- Messages
- 143
- Reaction score
- 66
A sample of monatomic ideal gas is taken through an adiabatic expansion and is then isothermally compressed until the gas returns to its original pressure. Which of the following is true of this process?
a) The net heat input into the gas is zero.
b) The net work done by the gas is zero.
c) The final state of the gas has a lower total internal energy than the initial state.
d) The final state of the gas has a higher average temperature than the initial state.
My diagram:
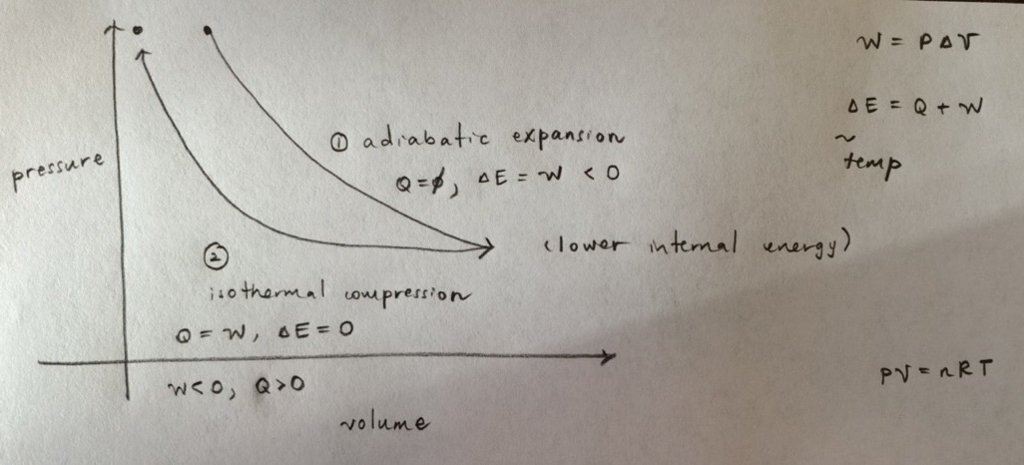
Thermo always confuses me. Can anyone correct me on my reasoning?
a) In the first adiabatic step, the heat input is 0. The gas does work in expanding and total energy decreases.
In the second isothermal step, the work is negative since volume decreases. So heat must be added to the system to keep the total energy the same.
b) Work done by the gas only occurs in the first step and is negative. I think the work done in the second step is by the system (on the gas).
c) I think this is the true statement. Energy is lost in the first adiabatic step and conserved in the isothermal step, so the total internal energy should be lower.
d) For an ideal gas, we know that PV = nRT applies. The final pressure is the same, and the final volume is lower, and since n and R are constant, the final temperature must also be lower.
a) The net heat input into the gas is zero.
b) The net work done by the gas is zero.
c) The final state of the gas has a lower total internal energy than the initial state.
d) The final state of the gas has a higher average temperature than the initial state.
My diagram:
Thermo always confuses me. Can anyone correct me on my reasoning?
a) In the first adiabatic step, the heat input is 0. The gas does work in expanding and total energy decreases.
In the second isothermal step, the work is negative since volume decreases. So heat must be added to the system to keep the total energy the same.
b) Work done by the gas only occurs in the first step and is negative. I think the work done in the second step is by the system (on the gas).
c) I think this is the true statement. Energy is lost in the first adiabatic step and conserved in the isothermal step, so the total internal energy should be lower.
d) For an ideal gas, we know that PV = nRT applies. The final pressure is the same, and the final volume is lower, and since n and R are constant, the final temperature must also be lower.
