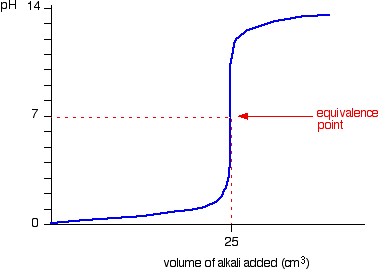
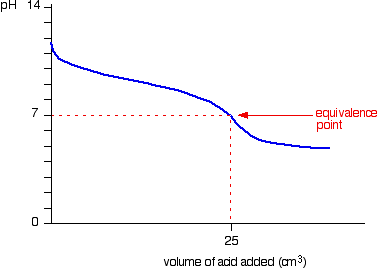
TBR has a good page explaining this mathematically but I don't understand very well.
They give an example and compare different conditions. This is the first:
Initially A- / HA = 1 / 999. After adding one part OH, then it becomes 2 / 998.
Because Ka = [H+] * [A-] / [HA], and Ka is constant, then [A-] / [HA] doubles, [H+] must be cut in half. pH changes and thus this is not a buffer.
First of all, i'm not understanding what is being said here. Second, "[H+] must be cut in half"; should it be equal to [A-]?
thank you!
They give an example and compare different conditions. This is the first:
Initially A- / HA = 1 / 999. After adding one part OH, then it becomes 2 / 998.
Because Ka = [H+] * [A-] / [HA], and Ka is constant, then [A-] / [HA] doubles, [H+] must be cut in half. pH changes and thus this is not a buffer.
First of all, i'm not understanding what is being said here. Second, "[H+] must be cut in half"; should it be equal to [A-]?
thank you!