- Joined
- Feb 11, 2008
- Messages
- 482
- Reaction score
- 206
Just out of curiosity, will we have to know some of the IUPAC/systematic names of certain drugs? The above is for Ciprofloxacin.
Just out of curiosity, will we have to know some of the IUPAC/systematic names of certain drugs? The above is for Ciprofloxacin.
what's that? It's a Pro at Ci? See Ipro!? C-I-P-R-O?There's a lot you can tell about cipro by looking at that name though...
what's that? It's a Pro at Ci? See Ipro!? C-I-P-R-O?
I almost crapped in my pants when i saw the name of this thread....
just 1 diet pepsi! I got the right one baby...How much have you been drinking tonight??

You're going to NSU right? No you won't...the curriculum is far more clinical-based. So you'll know everything about quinolones in that regard, but IUPAC naming won't be in the picture.
she looked at your posting history...never give a woman any creditExcellent! good memory! You remember I'm going to attend NSU this fall. Now that i'm at it, what was your first week of study at NSU like? If i'm not mistaken, the program doesn't offer an actual course in medicinal chemistry, but elements of this course are incorporated in pharmcodynamics...am i correct? How much different are the pharmacy textbooks compared to undergrad?
Excellent! good memory! You remember I'm going to attend NSU this fall. Now that i'm at it, what was your first week of study at NSU like? If i'm not mistaken, the program doesn't offer an actual course in medicinal chemistry, but elements of this course are incorporated in pharmcodynamics...am i correct? How much different are the pharmacy textbooks compared to undergrad?
Man you guys are lucky, here we have IUPAC naming on every Organic Chem test, and in almost every fashion.. drawing the structure from the name and naming the structure etc
I'd much rather learn laymen terms, (which we do on a really small scale) but I don't really have a choice in the matter
Hmm... Organic chem =/ Pharmacy school. We all did chemistry courses which require IUPAC... this is for pharmacy school courses.
P.S. How can you be a graduate in 2012, 6 year program or something?
Hmm... Organic chem =/ Pharmacy school. We all did chemistry courses which require IUPAC... this is for pharmacy school courses.
P.S. How can you be a graduate in 2012, 6 year program or something?
I would love to be in Organic Chem again compared to this Med Chem crap.....
I guess it depends on the school, but I love Med Chem!Cycloketocaine, wouldn't medicinal chemistry be even more interesting than organic chemistry? After all, i believe it is more applicable to the human body. So, why is it crap?


I guess it depends on the school, but I love Med Chem!
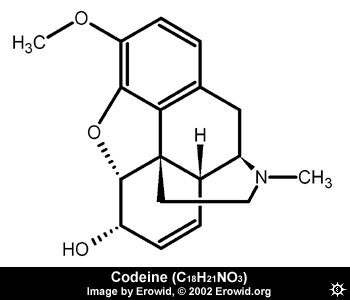
The same goes for Codeine and Morphine. Morphine is more active than Codeine, because it has an additional OH group.
10% of Codeine is metabolized to MorphineHeh. I've never heard that before, med chem here was terrible and completely irrelevant, with med chem making up 0% of the national licensing exam.
P.S. Codeine isn't more active than Morphine because of a hydroxyl group; it's a prodrug for morphine so only a fraction of codeine becomes morphine.. hence you give larger doses.
Also, I remember one or two med chem questions. That kind of freaked me out since everyone always said med chem wasn't on the exam.
I guess it depends on the school, but I love Med Chem!
If you understand why various parts of a structure are significant, you'll know more about the drug. I guess you could say that you'll know more than "it's for pain", "it's for anxiety", or "it's for infection". You'll know why it's believed that certain compounds do a better job.
For example:
Let's compare Oxycodone and Hydrocodone. After you analyze the structures, you should be able to notice that the only difference between Oxycodone and Hydrocodone is that Oxycodone has an additional OH (hydroxyl) group between the non-aromatic rings (hence, Oxycodone). OH groups increase activity --> Oxycodone is more active than Hydrocodone. The same goes for Codeine and Morphine. Morphine is more active than Codeine, because it has an additional OH group.
Hydrocodone
Morphine
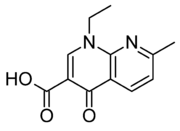
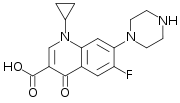
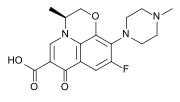
Let's compare quinolones and fluroquinolones. Halocarbons are lipophilic --> they have better penetration through cellular membranes. The F (fluoride) group on Ciprofloxacin increase it's lipophilicity.
The nitrogen groups (heterocyclic ring) that are substituted on the aromatic ring increase the drugs' activity spectrum. The cyclic nitrogen group attached to the non-aromatic ring of Ciprofloxacin works the same way --> increases activity spectrum.
Therefore... Ciprofloxacin and Levofloxacin have increased activity --> "better" antibiotics.
Nalidixic Acid (quinolone)
Ciprofloxacin (fluroquinolone)
Levofloxacin (fluorquinolone)
I have to go study, so that's all for today's chemistry lesson.

10% of Codeine is metabolized to Morphine
I read somewhere that there were a few Med Chem questions on the Naplex. Let me try to find where they said that...
thread
Are you trying to be cute?Hence I said a fraction
We don't write the NAPLEX in Canada.. it's the PEBC's + OSCE's.
I guess it depends on the school, but I love Med Chem!
If you understand why various parts of a structure are significant, you'll know more about the drug. I guess you could say that you'll know more than "it's for pain", "it's for anxiety", or "it's for infection". You'll know why it's believed that certain compounds do a better job.
For example:
Let's compare Oxycodone and Hydrocodone. After you analyze the structures, you should be able to notice that the only difference between Oxycodone and Hydrocodone is that Oxycodone has an additional OH (hydroxyl) group between the non-aromatic rings (hence, Oxycodone). OH groups increase activity --> Oxycodone is more active than Hydrocodone. The same goes for Codeine and Morphine. Morphine is more active than Codeine, because it has an additional OH group.
.
My Med Chem exam is tomorrow morning. I just love going to school on a Saturday.I just took my med chem final over that stuff. Can't say that I sad that it is over.
Med Chem at my school is useless and you can tell becuase the professors are too lazy to write new test questions (which is a God send in so many way)
Are you trying to be cute?
I didn't want to bring up prodrug to someone who probably has no concept of what that means. For the sake of discussion though, you're 100% correct. Codeine is a prodrug of morphine, and 1/10th (a fraction) of codeine is converted into morphine.
For anyone who's interested... this is a decent definition of a prodrug.

The same goes for Codeine and Morphine. Morphine is more active than Codeine, because it has an additional OH group.
The acetylated groups on heroine make the structure more lipophilic. This allows the structure to cross the blood brain barrier more rapidly than morphine. If taken orally, our bodies will deactylate heroine into morphine. If the acetylated version of morphine (heroine) is injected, it will remain acetylated in the body, it will not be affected by deactylation metabolism (first-pass metabolism), and this will lead to a greater analgesic effect.I really like your lecture......So why is it that when you acetylate both hydroxyl groups on morphine you get something way more potent than morphine??
