- Joined
- Jul 24, 2008
- Messages
- 218
- Reaction score
- 0
Can someone put the meaning of diastereomers, enantiomers, meso compounds, constitutional isomers and anything else of that sort in layman's terms? Thanks!

Can someone put the meaning of diastereomers, enantiomers, meso compounds, constitutional isomers and anything else of that sort in layman's terms? Thanks!
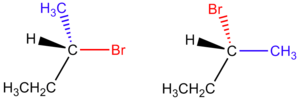
nice nze! well done.
question tho... say you have a cis 1,2 dichlorocyclopentane. That's a meso compound and its optically inactive.
What about a trans 1,2 dicholorcyclopentane. There is also a plane of symmetry there, correct? Does that make it optically inactive as well?
First part is tru. 2nd false. check out teh attachment. its hsould be optically active.
nice nze! well done.
question tho... say you have a cis 1,2 dichlorocyclopentane. That's a meso compound and its optically inactive.
What about a trans 1,2 dicholorcyclopentane. There is also a plane of symmetry there, correct? Does that make it optically inactive as well?
Thanks for the explanation guys... Just to sum things up, Lets say you have a compound with two chiral centers - 1R, 2R
The enantiomer of this compound is 1S, 2S
The diastereomers of this compound are 1R, 1S and 1S, 2R?
awesome, thanks. yeahh... I was just giving a random example actually. While we're on the stereochemistry topic, just as practice, in the two examples posted by razblo, the first one is S and the second is R, correct?
