- Joined
- Jul 13, 2011
- Messages
- 104
- Reaction score
- 51
Hey guys, was hoping for some help here. I took ochem this spring but I'm a bit rusty so here it is:
1.)
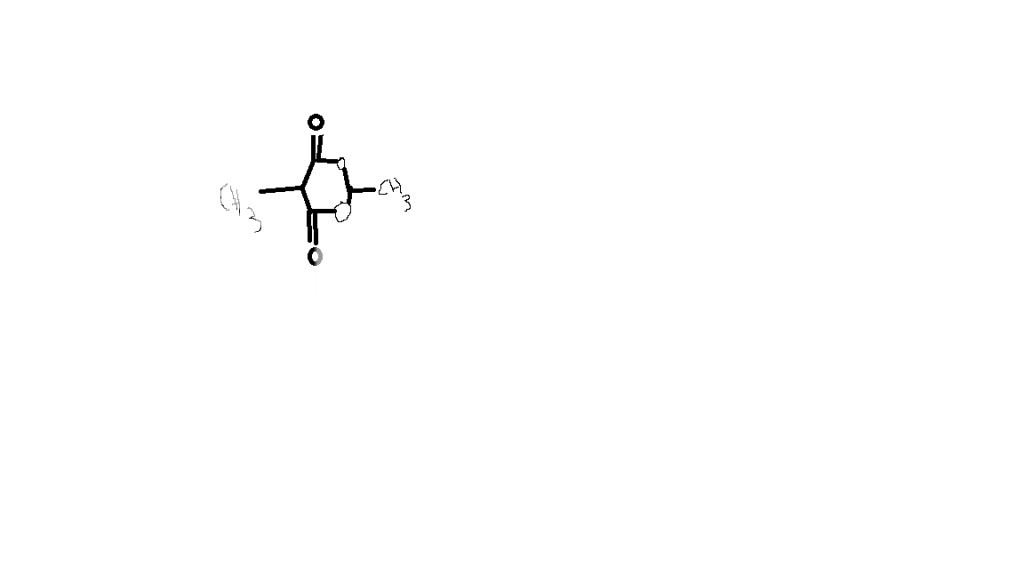
(Sorry for the tacky paint image... didn't know what this molecule's name is)
Anywho, I was asked what kind of molecule this is. Ester immediately popped into my mind-- and it was right-- but what confused me is why this molecule can't be considered an ether too. R O R is an ester, so wouldn't it count here?
Or is that designation not given when an aforementioned R is a carbonyl carbon?
2.) I was brushing up on the definition of being conjugated and came across this issue.
An amide is evidently conjugated. So from what I understand when nitrogen is part of, say, a ring system where it is double bonded on one side, with a single bond on the other, and has a lone pair, it is sp2 (given there is another atom that is also Sp2 part of that ring) and thus conjugated with that atom.
So my question is: that lone pair which normally makes the amine basic-- is it NOT going to react as a lewis base because it helps to make the molecule conjugated?
By extension then: if nitrogen had 2 single bonds to 2 carbons, and 1 bond to a hydrogen with a lone pair-- would it then be basic (as donating its electrons would make it conjugated)? If so, then is it the electronegativity of the carbonyl carbon that makes amides not as basic as they should be?
Now my question for the amide part: Because the nitrogen is sp3, would it donate its lone pair to become conjugated with the carbonyl carbon? If so, why are amides
3.) Which is more polar, an amide or alcohol? I'd appreciate an explanation on this if possible.
1.)

(Sorry for the tacky paint image... didn't know what this molecule's name is)
Anywho, I was asked what kind of molecule this is. Ester immediately popped into my mind-- and it was right-- but what confused me is why this molecule can't be considered an ether too. R O R is an ester, so wouldn't it count here?
Or is that designation not given when an aforementioned R is a carbonyl carbon?
2.) I was brushing up on the definition of being conjugated and came across this issue.
An amide is evidently conjugated. So from what I understand when nitrogen is part of, say, a ring system where it is double bonded on one side, with a single bond on the other, and has a lone pair, it is sp2 (given there is another atom that is also Sp2 part of that ring) and thus conjugated with that atom.
So my question is: that lone pair which normally makes the amine basic-- is it NOT going to react as a lewis base because it helps to make the molecule conjugated?
By extension then: if nitrogen had 2 single bonds to 2 carbons, and 1 bond to a hydrogen with a lone pair-- would it then be basic (as donating its electrons would make it conjugated)? If so, then is it the electronegativity of the carbonyl carbon that makes amides not as basic as they should be?
Now my question for the amide part: Because the nitrogen is sp3, would it donate its lone pair to become conjugated with the carbonyl carbon? If so, why are amides
3.) Which is more polar, an amide or alcohol? I'd appreciate an explanation on this if possible.
