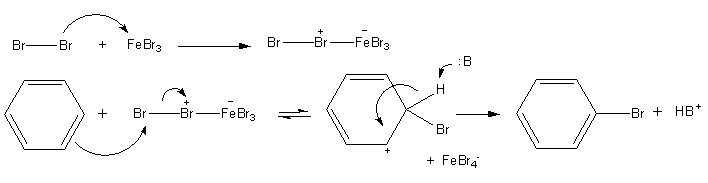
A compound to be analyzed is known to be a six carbon cyclic hydrocarbon. The compound is found to be inert to bromine in water and to bromine in dichloromethane, yet it decolorizes bromine in carbon tetrachloride when a small quantity if FeBr3 is added. Which of the following could be the identity of the compound?
a. cyclohexane OUT
b. benzene Edit!! It's benzene!!
c. 1,3 cyclohexadiene
d. 1,4 cyclohexadiene
Can someone explain?
All I know is "decolorizes bromine in carbon tetrachloride" = means alkene and that's how I eliminated choice a & b.
a. cyclohexane OUT
b. benzene Edit!! It's benzene!!
c. 1,3 cyclohexadiene
d. 1,4 cyclohexadiene
Can someone explain?
All I know is "decolorizes bromine in carbon tetrachloride" = means alkene and that's how I eliminated choice a & b.
Last edited: