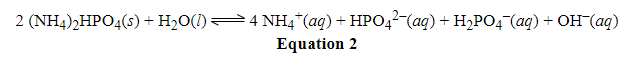This one is actually quite complicated and, in my opinion, the AAMC has it wrong. Usually, water does not appear in equilibrium expressions because it has an activity of one and it's actually activities that appear in the equilibrium expression. In other words, it has the same concentration (55.5 M) as the standard state of water: activity = [X]/[X in the standard state]. This applies for situations where water is the solvent in a chemical reaction or process. However, in some cases, water will also act as a reactant or product. In those cases, whether water will appear in the equilibrium expression is more nuanced. In most cases, the amount of water consumed or produced is negligible relative to the amount of solvent that's there. Remember that there are 55.5 moles of water per liter of it - that's a whole lot! So for most reactions, the amount of water consumed or produced is quite small and doesn't make a dent in the water concentration, which in turn doesn't make a dent in the activity. Therefore, we just ignore water in those cases. That's what I believe we should do here.
In some cases, your solvent isn't water and water serves only as a reactant. If the water is dissolved in the other solvent, then its concentration will be very different from its standard state and its activity will not be 1. Therefore, it will contribute to the equilibrium expression and thus to the equilibrium.