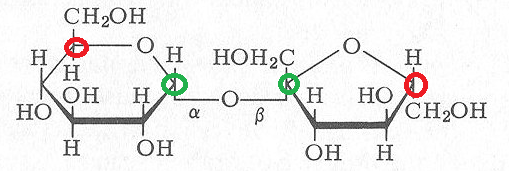
I'm confused at the labeling of alpha/beta linkage on SUCROSE. I've searched the threads, and still am realllly confused. According to TBR, alpha = when anomeric oxygen and last carbon are opposite (one up, one down); and beta = same side.

For the sucrose above, the glucose on the left is alpha because the anomeric carbon's O is opposite side of the CH2OH. The issue is fructose -- where is the anomeric carbon? Can someone label or let me know what i should be looking for to determine alpha or beta, or where the heck the anomeric oxygen if so fructose. there appears to be an anomeric H, but the O is gone..

For the sucrose above, the glucose on the left is alpha because the anomeric carbon's O is opposite side of the CH2OH. The issue is fructose -- where is the anomeric carbon? Can someone label or let me know what i should be looking for to determine alpha or beta, or where the heck the anomeric oxygen if so fructose. there appears to be an anomeric H, but the O is gone..
Last edited: