- Joined
- Mar 15, 2011
- Messages
- 912
- Reaction score
- 988
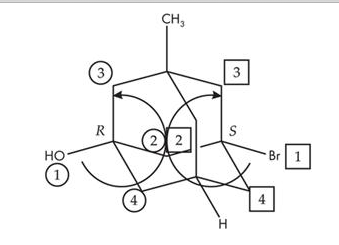
The image is from TPR's Demo Exam Q17.
I do not understand how to go about figuring out which group are "towards" or "away" from you when in an cyclical ring like this. I get assigning priorities, but counterclockwise wouldn't lead to R like on the left side of the molecule... so how do I know when to use the "reverse" rule??

