- Joined
- Oct 21, 2016
- Messages
- 689
- Reaction score
- 664
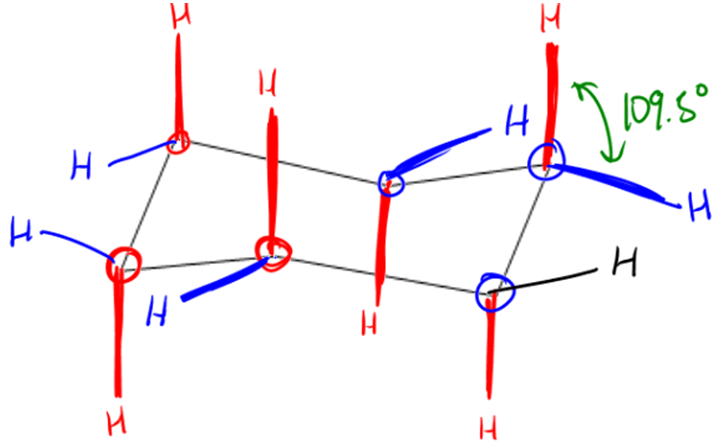
In BR I'm having a tough time with this concept. It says "Within a staggered conformation of Butane with CH3 groups, Anti is considered the most stable structure over its counterpart Gauche." I understand that steric hindrance is why it is considered the "most stable." But I am currently reading about Cyclohexane. So three- and four-membered rings are reactive, while five- and six-membered rings are considered stable. Well in Cyclohexane it iterates that chair conformation offers two substituent positions: equatorial and axial, and equatorial is considered more stable than axial. But here is my question, since axial looks similar to the staggered Anti compound, how is it not considered more stable? Is it because it can form more hydrogen bonds?