39 y/o with triple-negative breast cancer, 1.9 cm in size, had lumpectomy after neoadjuvant chemo which showed complete response. I'm treating whole breast to 42.6/16. Would anyone omit boost? Thanks.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Breast boost after complete response to neoadjCT
- Thread starter seper
- Start date
- Joined
- Apr 21, 2011
- Messages
- 3,744
- Reaction score
- 9,565
I wouldn't omit.
- Joined
- Mar 20, 2013
- Messages
- 2,187
- Reaction score
- 4,099
Boost.
I would not de-escalate in a young patient with TN disease like this.
I don't think it's wrong per se to consider omitting boost, I just personally wouldn't do this.
I would not de-escalate in a young patient with TN disease like this.
I don't think it's wrong per se to consider omitting boost, I just personally wouldn't do this.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
As is, the evidence for boosting the cavity in a Stage I patient getting hypofx is mighty, mighty weak. Why did this even ever get to be a thing, boosting cavities w/ hypofractionation? Tradition! You can pry standard fractionation from me but you'll take tumor cavity boosts over my dead body. In addition, the patient had a CR to chemo. This puts her form of triple-negative disease, natural history wise, outside typical bad prognosis triple negative disease. Like in the major trials testing hypofx vs standard fx, I never add a boost (because they didn't in the trials, eg START and Whelan). In summary your honor, there is no randomized data to support adding a boost here and extensive evidence that says a pCR is very predictive of improved prognosis (including LR risk) for her. In addition, the reason hypofractionation in breast is associated with slightly better cosmesis is because the total dose delivered was BED equivalent to ~50/25 for α/β ≅ 3 (50*1.67=83, 42.5*1.9=81). This assumption and expectation for equal and/or no worse side effects is completely eliminated if you are a hypofractionator and breast booster.
Last edited:
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
Boost.
(I usually look for reasons not to boost. But this one ain't it, chief)
(I usually look for reasons not to boost. But this one ain't it, chief)
- Joined
- Mar 20, 2013
- Messages
- 2,187
- Reaction score
- 4,099
As is, the evidence for boosting the cavity in a Stage I patient getting hypofx is mighty, mighty weak. Why did this even ever get to be a thing, boosting cavities w/ hypofractionation? Tradition! You can pry standard fractionation from me but you'll take tumor cavity boosts over my dead body. In addition, the patient had a CR to chemo. This puts her form of triple-negative disease, natural history wise, outside typical bad prognosis triple negative disease. Like in the major trials testing hypofx vs standard fx, I never add a boost (because they didn't in the trials, eg START and Whelan). In summary your honor, there is no randomized data to support adding a boost here and extensive evidence that says a pCR is very predictive of improved prognosis (including LR risk) for her. In addition, the reason hypofractionation in breast is associated with slightly better cosmesis is because the total dose delivered was BED equivalent to ~50/25 for α/β ≅ 3 (50*1.67=83, 42.5*1.9=81). This assumption and expectation for equal and/or no worse side effects is completely eliminated if you are a hypofractionator and breast booster.
Of note, in the Lyon boost trial I believe the whole breast dose was 50 Gy in 20 fractions, so sort of hypofractionated a little bit. I also recall about 50% of patients in START A/B getting a boost too - but would need to check on that.

Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France - PubMed
Delivery of a boost of 10 Gy to the tumor bed after 50 Gy to the whole breast following limited surgery significantly reduces the risk of early local recurrence, with no serious deterioration in the cosmetic result. Additional follow-up evaluation will be required to assess the long-term results.
Obviously only Lyon tested the role of boost, but a big chunk of START A/B patients had a boost in both arms though it appears.
Last edited:
- Joined
- Aug 6, 2013
- Messages
- 425
- Reaction score
- 713
Like in the major trials testing hypofx vs standard fx, I never add a boost (because they didn't in the trials, eg START and Whelan).
This is not quite true. 61% of START patients received a boost (10/5), which is why, when I choose to boost, I only use START fractionation (40/15) in order to be most in line with available data. They also stratified by boost.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Of note, in the Lyon boost trial I believe the whole breast dose was 50 Gy in 20 fractions, so sort of hypofractionated a little bit. I also recall about 50% of patients in START A/B getting a boost too - but would need to check on that.

Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France - PubMed
Delivery of a boost of 10 Gy to the tumor bed after 50 Gy to the whole breast following limited surgery significantly reduces the risk of early local recurrence, with no serious deterioration in the cosmetic result. Additional follow-up evaluation will be required to assess the long-term results.www.ncbi.nlm.nih.gov
Obviously only Lyon tested the role of boost, but a big chunk of START A/B patients had a boost in both arms though it appears.
Yes there are exceptions to every rule; never thought these hypofractionation trials would be cited as reason for boosting Stage I. You cited START but START said:This is not quite true. 61% of START patients received a boost (10/5), which is why, when I choose to boost, I only use START fractionation (40/15) in order to be most in line with available data. They also stratified by boost.
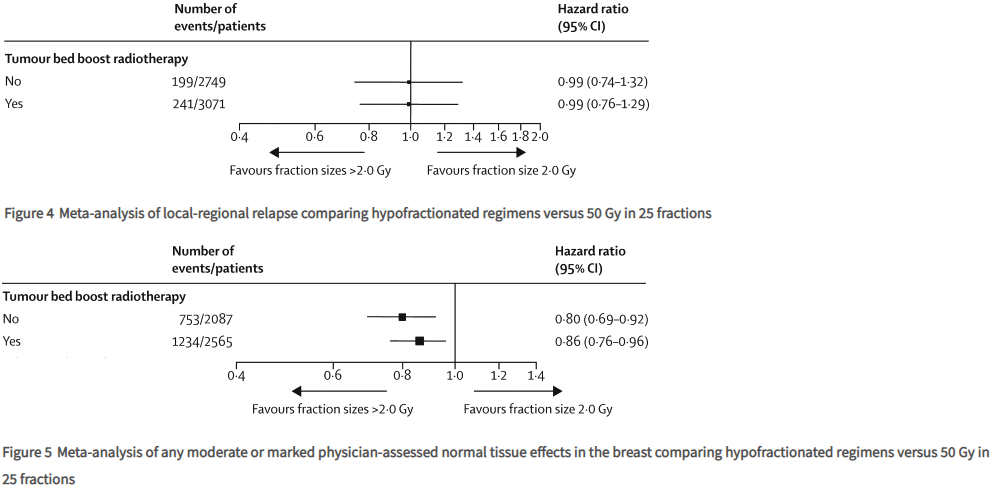
Post-hoc subgroup analyses of the combined hypofractionated regimens versus the control groups for local-regional relapse in START-A, START-B, and the pilot trial (n=5861) showed that the treatment effect was not significantly different irrespective of age, type of primary surgery, axillary node status, tumour grade, adjuvant chemotherapy use, or use of tumour bed boost radiotherapy.
So why boost? Hasn't it been "proven" to be ineffective here? (Maybe that's why I don't boost in hypofx.)
Was triple negative associated with increased LRR in Whelan or START?
pCR in triple negative decreases local recurrence risk to a greater magnitude than does XRT.
You guys are obsessed with LRR in breast cancer! All available data says her in-cavity recurrence risk is ~5%, and will be less than that with a pCR. AND she has triple negative disease and you're concerned about her cavity recurrence risk. What if her tumor cavity is right over her heart? She's 39.
There may have been a large component of breast boosting in START (I don't think there was in Whelan at all), but there was also a large component of T1N0 patients in the EORTC RNI trial. And if her tumor had been 2 millimeters larger she might've fit into MA20. I guess I should be thankful you guys aren't stridently pushing for RNI here...
I'm open to learning but AFAIK there is zero evidence of boost improving outcomes in hypofractionation.
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
Scarbtj. What are you talking about. You said the hypofrac trials didn’t boost and then you were shown that the majority of patients in THE large hypofrac trial patients got boosted. This isn’t an exception to the rule lol.
Then you show that the treatment effect wasn’t impacted by the boost - what this means is whether or not they got the boost, hypofrac was associated with the same local control compared to standard frac. This does NOT mean that for an individual patient that a boost does not improve their local control. This patient has young age and a high grade, both of which were born out as important factors for benefiting from Boost in the EORTC trial. Other studies have shown over and over again that triple negative histology is a risk factor for local failure.
It’s a fairly easy decision to boost.
Then you show that the treatment effect wasn’t impacted by the boost - what this means is whether or not they got the boost, hypofrac was associated with the same local control compared to standard frac. This does NOT mean that for an individual patient that a boost does not improve their local control. This patient has young age and a high grade, both of which were born out as important factors for benefiting from Boost in the EORTC trial. Other studies have shown over and over again that triple negative histology is a risk factor for local failure.
It’s a fairly easy decision to boost.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Explain how boost/no boost not being associated with local recurrence risk "does NOT mean that for an individual patient that a boost does not improve their local control"; I don't get it. And was age or grade assoc w/ increased LRR in the hypofx trials? I also asked was triple neg? I *do* know pCR in triple neg is significantly associated with LR risk (decrease). I get the "gut feelings"--she's young! She's triple neg! She's high grade! You can't omit a boost! It's just all the data points away from, rather than toward, these being significant in this scenario of Stage I triple neg with pCR as it pertains to she needs, or does not need, a much higher RT dose to her tumor cavity. To me, all this will add is toxicity.Then you show that the treatment effect wasn’t impacted by the boost - what this means is whether or not they got the boost, hypofrac was associated with good local control compared to standard frac. This does NOT mean that for an individual patient that a boost does not improve their local control.
Last edited:
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
The treatment effect (aka the impact of hypofrac) was consistent despite any patient or disease related factor in the Start trial. Which is to say ‘there is no subset in which hypo-fractionation did not work and was inferior to standard frac’. Which is what leads to the ASTRO consensus now. You can hypofrac anyone
This is different from ‘patients getting hypofrac don’t benefit from a boost’
That’s an illogical leap to make
This is different from ‘patients getting hypofrac don’t benefit from a boost’
That’s an illogical leap to make
- Joined
- Aug 6, 2013
- Messages
- 425
- Reaction score
- 713
Its a pretty easy conclusion to draw that the thoughtful UK doctors following their evidence based guidelines only boosted the younger higher grade patients, which ultimately supports the use of boost as these more unfavorable patients subsequently had the same local failure risks as their more favorable counterparts at long term follow up. Hooray for boost!
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Leave hypofx out of it. I don't know if I'm being obtuse or you are. Use of boost was not associated with LR risk in START, T or F? If T, why is it "illogical" to say boost does not affect LR risk?The treatment effect (aka the impact of hypofrac) was consistent despite any patient or disease related factor in the Start trial. Which is to say ‘there is no subset in which hypo-fractionation did not work and was inferior to standard frac’. Which is what leads to the ASTRO consensus now. You can hypofrac anyone
This is different from ‘patients getting hypofrac don’t benefit from a boost’
That’s an illogical leap to make
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
AKAIts a pretty easy conclusion to draw that the thoughtful UK doctors following their evidence based guidelines only boosted the younger higher grade patients, which ultimately supports the use of boost as these more unfavorable patients subsequently had the same local failure risks as their more favorable counterparts at long term follow up. Hooray for boost!
"Because the patients in the group who received the treatment had no beneficial effect from the treatment, clearly the treatment is beneficial."
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
This point of data supports your point.
However, it was clinician selection on who got boosts and who didn’t, so it’s hard to make a firm statement from this. It’s pretty spurious as radonc monkey says. But sure I agree that you can use this data if you wish.
However, it was clinician selection on who got boosts and who didn’t, so it’s hard to make a firm statement from this. It’s pretty spurious as radonc monkey says. But sure I agree that you can use this data if you wish.
Last edited:
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
The whole raison d'etre of breast hypofractionation: treat in as few fractions as possible. That is the game. The game is not improve LRR outcomes; the outcomes are already too good. It's sure not to improve OS. And the game is also to keep the side effects equal-to-better. But mostly equal. The game is also cost lowering and convenience.This point of data supports your point.
However, it was clinician selection on who got boosts and who didn’t, so it’s hard to make a firm statement from this. It’s pretty spurious as radonc monkey says. But sure I agree that you can use this data if you wish.
So a breast boost, which has no beneficial evidentiary basis whatsoever in hypofx (I think?), violates the rules of the game. It extends treatment by ~25%, adds substantial cost, and potentially adds toxicity. All without tangible benefit.
Which means we are a neurotic specialty. Present company included!
- Joined
- Aug 6, 2013
- Messages
- 425
- Reaction score
- 713
Leave hypofx out of it. I don't know if I'm being obtuse or you are. Use of boost was not associated with LR risk in START, T or F? If T, why is it "illogical" to say boost does not affect LR risk?
To me, its kind of like saying, well we are testing this new chemo regimen, and we have these HER2+ patients who we gave Herceptin and Perjeta to as it is standard of care, and they didn't do any better or worse that these other HER2 negative patients, so I can't prove that the anti HER2 therapy did anything. Seems disingenuous, no?
I agree that as a field we over-boost people. However, I disagree with your interpretation of the data that boosting has no effect in hypofractionation. The START patients received a boost because they were high risk (the exact population that was shown to benefit from boost) and it appears the boost negated that risk. The trial was not randomized for boost. No conclusions can be made as a result and therefore you must rely on the data that originally established the local control benefit of boost.
Do you want these trial repeated in the setting of hypofractionation? Probably not gonna happen. I will continue boosting high grade and young patients as is the national standard and in all guidelines.
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
Scar - I mean you’re preaching to the choir - I really don’t boost many people. I think in many cases it’s just an excuse for an extra billable on treatment visit and five extra fractions of pay.
A 39 year old triple negative patient is probably what it takes for me to recommend a boost. At the end of the day - if 40/15 is the same biologically as 50/25 - then you should boost someone a bit higher if you are worried enough, no matter which biological initial dose they had?
But we are talking about something that ultimately isn’t that important. Boost is ultimately ‘chump change’. This is probably just someone I would personally boost.
A 39 year old triple negative patient is probably what it takes for me to recommend a boost. At the end of the day - if 40/15 is the same biologically as 50/25 - then you should boost someone a bit higher if you are worried enough, no matter which biological initial dose they had?
But we are talking about something that ultimately isn’t that important. Boost is ultimately ‘chump change’. This is probably just someone I would personally boost.
- Joined
- Sep 20, 2004
- Messages
- 12,365
- Reaction score
- 12,842
I do everything and the kitchen sink for TNBC, including offering pmrt to young patients with T1/T2N0 disease.Scar - I mean you’re preaching to the choir - I really don’t boost many people. I think in many cases it’s just an excuse for an extra billable on treatment visit and five extra fractions of pay.
A 39 year old triple negative patient is probably what it takes for me to recommend a boost. At the end of the day - if 40/15 is the same biologically as 50/25 - then you should boost someone a bit higher if you are worried enough, no matter which biological initial dose they had?
But we are talking about something that ultimately isn’t that important. Boost is ultimately ‘chump change’. This is probably just someone I would personally boost.
Also data for adjuvant xeloda if this lady had residual disease at surgery.... https://www.nejm.org/doi/10.1056/NEJMoa1612645
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
"I know what I know, and there's no data that I'm wrong." True. All we have is data from the hypofx trials (Whelan, START) where "risk"... grade, age, etc... were not really associated with LR outcomes. No one has yet even touched on the fact that she was a pCR and what a profound piece of data for this patient re: recurrence risk that is. It actually makes her, a Stage I patient with pCR, low recurrence risk. (There are still folks thinking that HER2+ is high risk; it's not in this era, but it was in previous eras.) Guidelines only recommend boosts for patients with high recurrence risks. Guidelines are fallible! So we are left with gut feelings. Gut feelings inhibit free thought or at least the ability to hold two conflicting thoughts in one's mind at the same time. The feelings on the flipside of that coin... "I am possibly over-irradiating"... are seldom explored. Perhaps OP's patient is a non-exerciser. There's vastly more data that exercise will affect this patient's LR risk than a breast boost. So my recommendation would be: if one is going to boost this patient, at least also put her on an exercise program.To me, its kind of like saying, well we are testing this new chemo regimen, and we have these HER2+ patients who we gave Herceptin and Perjeta to as it is standard of care, and they didn't do any better or worse that these other HER2 negative patients, so I can't prove that the anti HER2 therapy did anything. Seems disingenuous, no?
I agree that as a field we over-boost people. However, I disagree with your interpretation of the data that boosting has no effect in hypofractionation. The START patients received a boost because they were high risk (the exact population that was shown to benefit from boost) and it appears the boost negated that risk. The trial was not randomized for boost. No conclusions can be made as a result and therefore you must rely on the data that originally established the local control benefit of boost.
Do you want these trial repeated in the setting of hypofractionation? Probably not gonna happen. I will continue boosting high grade and young patients as is the national standard and in all guidelines.
You got it. EDIT: look for more of this thinking once APM is a reality 🙂Boost is ultimately ‘chump change’.
Last edited:
- Joined
- Oct 10, 2011
- Messages
- 8,918
- Reaction score
- 11,385
I actually kind of agree with scarb on this one. I think we all snap to boosting TNBC beceause of historical outcomes. However, as is well accepted, pCR to NAC in TNBC (I mean really in any non-Luminal A breast cancer) is a huge predictor of improved LRR.
While I would probably still boost with a 39-year old (after discussion of potential toxicities), I don't think it's unreasonable to skip the boost in somebody who has a pCR. Somewhat dependent on patient preferences IMO.
While I would probably still boost with a 39-year old (after discussion of potential toxicities), I don't think it's unreasonable to skip the boost in somebody who has a pCR. Somewhat dependent on patient preferences IMO.
- Joined
- Mar 20, 2013
- Messages
- 2,187
- Reaction score
- 4,099
I actually kind of agree with scarb on this one. I think we all snap to boosting TNBC beceause of historical outcomes. However, as is well accepted, pCR to NAC in TNBC (I mean really in any non-Luminal A breast cancer) is a huge predictor of improved LRR.
While I would probably still boost with a 39-year old (after discussion of potential toxicities), I don't think it's unreasonable to skip the boost in somebody who has a pCR. Somewhat dependent on patient preferences IMO.
B-18 and B-27 data give us some insight to local recurrence; doesn't exactly asnwer the +/- boost question.
For a young person, clinically node negative with a breast path CR risk of in breast failure seems to be around 7%. Not sure how many of these patients had boost (I presume most).
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Would you skip irradiating the IMNs and sclav in someone who was cT1N1 HER2+ and who was pT0N0 after TCHP chemo 🙂I actually kind of agree with scarb on this one. I think we all snap to boosting TNBC beceause of historical outcomes. However, as is well accepted, pCR to NAC in TNBC (I mean really in any non-Luminal A breast cancer) is a huge predictor of improved LRR.
While I would probably still boost with a 39-year old (after discussion of potential toxicities), I don't think it's unreasonable to skip the boost in somebody who has a pCR. Somewhat dependent on patient preferences IMO.
You say you might skip the tumor cavity boost; her LR risk there (low) is higher than her node relapse risk (quite low).
Sorry. But you did kind of make my RNI point heh heh, which was: consider biology first, stage/age/grade and that sort of thing a bit second. And TNBC is "bad." But as @BobbyHeenan shows above, TNBC w/ pCR (which is a particularly different biology) is actually pretty dang good. And this (B18/27) was with 1st gen chemo regimens.
- Joined
- Aug 6, 2013
- Messages
- 425
- Reaction score
- 713
I actually kind of agree with scarb on this one. I think we all snap to boosting TNBC beceause of historical outcomes. However, as is well accepted, pCR to NAC in TNBC (I mean really in any non-Luminal A breast cancer) is a huge predictor of improved LRR.
While I would probably still boost with a 39-year old (after discussion of potential toxicities), I don't think it's unreasonable to skip the boost in somebody who has a pCR. Somewhat dependent on patient preferences IMO.
Boosting in setting of hypofractionation and boosting in the setting of path CR are two different issues, neither with robust data to guide us. Although both may often (not always...) be pissing in the wind to some degree.
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
I mwan
This is sort of what B51 will answer. I suspect it will ultimately be okay to skip RNI in patients who clear node disease with chemo.
The combined data of B18 and 27 shows super low rates of LF in people who cleared the nodes at surgery (yN0) who did not get RT.
Would you skip irradiating the IMNs and sclav in someone who was cT1N1 HER2+ and who was pT0N0 after TCHP chemo 🙂
You say you might skip the tumor cavity boost; her LR risk there (low) is higher than her node relapse risk (quite low).
Sorry. But you did kind of make my RNI point heh heh, which was: consider biology first, stage/age/grade and that sort of thing a bit second. And TNBC is "bad." But as @BobbyHeenan shows above, TNBC w/ pCR (which is a particularly different biology) is actually pretty dang good. And this (B18/27) was with 1st gen chemo regimens.
This is sort of what B51 will answer. I suspect it will ultimately be okay to skip RNI in patients who clear node disease with chemo.
The combined data of B18 and 27 shows super low rates of LF in people who cleared the nodes at surgery (yN0) who did not get RT.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Indeed. Boosting with hypofractionation is pissing into a light gust. Boosting with hypofractionation and a pCR in Stage I? Pissing into a typhoon.Boosting in setting of hypofractionation and boosting in the setting of path CR are two different issues, neither with robust data to guide us. Although both may often (not always...) be pissing in the wind to some degree.
Going out on a limb and presuming 0.0% of the boost patients in B18/27 were hypofractionated 😉For a young person, clinically node negative with a breast path CR risk of in breast failure seems to be around 7%. Not sure how many of these patients had boost (I presume most).
Last edited:
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
I honestly don’t think the hypofrac has anything to do with it.
If you would boost this patient if you gave them 50/25, then it should not be any different if you do 40/15. Not different at all.
Scarb - I assume you do not boost anyone really in your practice?
If you would boost this patient if you gave them 50/25, then it should not be any different if you do 40/15. Not different at all.
Scarb - I assume you do not boost anyone really in your practice?
- Joined
- Dec 17, 2007
- Messages
- 3,702
- Reaction score
- 5,209
The fact that boost or no–boost had no impact on local recurrence rates does not mean that boost is worthless. It means that the UK doctors boosted the right patients (the high risk ones) and omitted the boost in the right patients (the low risk ones).
It‘s like saying „Carrying an umbrella or not has no significant impact on me staying dry“. If you watch the weather forecast daily and carry the umbrella on rainy days and leave the umbrella at home on sunny days, at the end of the year, you carrying an umbrellla or not will not have a significant impact on staying dry (statistically speaking). You will always be dry.
It‘s like saying „Carrying an umbrella or not has no significant impact on me staying dry“. If you watch the weather forecast daily and carry the umbrella on rainy days and leave the umbrella at home on sunny days, at the end of the year, you carrying an umbrellla or not will not have a significant impact on staying dry (statistically speaking). You will always be dry.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
I never ever never ever boost a hypofractionated patient. I have no (randomized) data to guide me as to whether it helps; the gestalt one gets is, as mentioned, is of no oncological benefit with some mild toxicity risk increase possible. Again, the "game" of hypofractionation is: improved convenience, decreased cost, equipoise in risk/benefit ratios to normofractionation. If I were doing a PowerPoint, I could make a pretty good talk (see everything in this thread above) about how breast boosting hypofx patients doesn't play by the rules of the hypofx game. Mentioning old breast boost data from normofractionation trials where boosting improved outcomes (I once asked Jay Harris around 2002, "so it seems like the breast boost data is a homerun?"... he said, "It's a solid double") is essentially rendered moot by all the data from the hypofx trials. Because the boost has been proven superior in normofractionation, I always boost when normofractionating a lumpectomy patient. It may rightly seem inconsistent, but at least I have good data to navigate that river. In Whelan's original report of breast hypofx reported in a randomized fashion, a boost of 12.5/5 on top of 40/16 was used. Then, in his next randomized trial, they skipped the boost altogether. Outcomes in this totally non-scientific comparison (another reason I don't boost hypofx):I honestly don’t think the hypofrac has anything to do with it.
If you would boost this patient if you gave them 50/25, then it should not be any different if you do 40/15. Not different at all.
Scarb - I assume you do not boost anyone really in your practice?
............................................................40/16+12.5/5......................42.5/16
Local recurrence risk...................11%@10y..............................6%@10y
Ergo, less local recurrence without a boost 😉 The extra 2.5Gy whole breast took care of it heh heh.
"If you would boost this patient if you gave them 50/25, then it should not be any different if you do 40/15. Not different at all." I see how you could see this if you could cite me any trials of boost/no boost in hypofx showing benefit to boost. I can of course cite several showing boost's benefit from normofractionation; this was always a LR benefit only at the risk of slightly worse toxicity.
How in the heck, ye lovers of boost in hypofx, does one boost in this situation? This, partial breast, is now my go-to for many Stage I patients.
(I enjoy these talks. They really make me think.)
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
I would like to be loaned their crystal ball.The fact that boost or no–boost had no impact on local recurrence rates does not mean that boost is worthless. It means that the UK doctors boosted the right patients (the high risk ones) and omitted the boost in the right patients (the low risk ones).
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
I really don’t understand the logic you’re using to say the data doesn’t support boost in hypofrac. Like at all. The umbrella analogy is a good one.
Also - You said yourself that 50/25
Is the same as 40/15.
Anyways it’s fine. We can agree to disagree.
Also - You said yourself that 50/25
Is the same as 40/15.
Anyways it’s fine. We can agree to disagree.
Last edited:
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
I think the CLOSEST we will ever get to understanding this is the fact that Whelan did not boost in his trial and that even in the long term update, high grade patients had a statistically significant three fold recurrence risk (16 percent local failure vs 5 for non grade 3). These patients need a higher dose, as we already knew from the EORTC boost trial.
Boom.
If you’re waiting for a randomized trial of +/- boost again in 2019, keep waiting.
In fact - RTOG 1005 had boosts in both arms.
Boom.
If you’re waiting for a randomized trial of +/- boost again in 2019, keep waiting.
In fact - RTOG 1005 had boosts in both arms.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
On the other hand...The fact that boost or no–boost had no impact on local recurrence rates does not mean that boost is worthless. It means that the UK doctors boosted the right patients (the high risk ones) and omitted the boost in the right patients (the low risk ones).
It‘s like saying „Carrying an umbrella or not has no significant impact on me staying dry“. If you watch the weather forecast daily and carry the umbrella on rainy days and leave the umbrella at home on sunny days, at the end of the year, you carrying an umbrellla or not will not have a significant impact on staying dry (statistically speaking). You will always be dry.
1) As oncologists, we have to try and predict things >10 days advance and wouldn't accept a weather predictor's success odds. We as humans are bad predictors, though, so better just to stick to actual study results vs "here's what I take from my own post-hoc non-mathematical analysis of the study." Too prone to validation bias.
2) Umbrellas are not benign.
3) Do umbrellas even keep you dry?
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
That high grade association was invalidated on later analysis I think?* And in fact, if we were to be data purists and stick with the trial arms, we would rephrase your supposition as:I think the CLOSEST we will ever get to understanding this is the fact that Whelan did not boost in his trial and that even in the long term update, high grade patients had a statistically significant three fold recurrence risk (16 percent local failure vs 5 for non grade 3). These patients need a higher dose, as we already knew from the EORTC boost trial.
Boom.
If you’re waiting for a randomized trial of +/- boost again in 2019, keep waiting.
In fact - RTOG 1005 had boosts in both arms.
"High grade patients had lower risk of local recurrence with normofractionation vs hypofractionation in Whelan." Thus an argument against hypofx, not for boost. No dose escalation inferences per se can be inferred.
* this supposedly: Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. - PubMed - NCBI
Last edited:
- Joined
- Mar 20, 2013
- Messages
- 2,187
- Reaction score
- 4,099
I posted this earlier but why ignore the Lyon trial? That was hypofractionation-ish at 50/20 whole breast +/- boost and showing a benefit.
The pCR throws it all for a loop though.
I still vote boost but on our weekly chart review if a partner elected for no boost I wouldn't say a word because I think it's a judgement call.
The pCR throws it all for a loop though.
I still vote boost but on our weekly chart review if a partner elected for no boost I wouldn't say a word because I think it's a judgement call.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
The Lyon trial (who knew this gave insight into hypofx? Who knew START gave insight into boosting?) is not ignore-able. It gave women a whole one percent improved local recurrence risk. EORTC's boost benefits were more pronounced, but its LR risks were in some cases much higher than what we see in routine practice today. And it was not "hypofractionated" like Lyon. At the end of the day, Lyon boost arm/EORTC boost arm goes up against Whelan(no boost)/START(no boost benefit) and the LR risks are all nestled tightly together. What we have w/hypofx boosting is more cost, more side effects, no measurable tangible benefits unless we extrapolate boost's effects from wildly different fractionation regimens... and eras. What could the benefit from boosting in hypofx be? In Lyon one got 1% improvement from boost, in EORTC about 4%. Think we can get 4% LR improvement when LR risks are ~6% with hypofx without boost? No way. Maybe 2%? On paper, giving 25% more dose and 25% more treatments to 100% of women to help one out of fifty of them (at best) avoid a local recurrence with no improvement in survival sounds crazy. I am not as good as the UK doctors who know exactly who and who not to boost with hypofx (and evidently the boost made no difference but let's not quibble on that), another detail I'm depressingly learning today. I would have to boost all hypofx patients to be sure it was working, because in all the hypofx trials no standard risk factor (grade, age, LVI, not even nodes, etc.) could predict who was at higher risks of recurrence. I couldn't be any better predictor than the trials' data.I posted this earlier but why ignore the Lyon trial? That was hypofractionation-ish at 50/20 whole breast +/- boost and showing a benefit.
The pCR throws it all for a loop though.
I still vote boost but on our weekly chart review if a partner elected for no boost I wouldn't say a word because I think it's a judgement call.
Your chart reviews sound pretty reasonable btw.
Last edited:
- Joined
- Aug 6, 2013
- Messages
- 425
- Reaction score
- 713
The Lyon trial (who knew this gave insight into hypofx? Who knew START gave insight into boosting?) is not ignore-able. It gave women a whole one percent improved local recurrence risk. EORTC's boost benefits were more pronounced, but its LR risks were in some cases much higher than what we see in routine practice today. And it was not "hypofractionated" like Lyon. At the end of the day, Lyon boost arm/EORTC boost arm goes up against Whelan(no boost)/START(no boost benefit) and the LR risks are all nestled tightly together. What we have w/hypofx boosting is more cost, more side effects, no measurable tangible benefits unless we extrapolate boost's effects from wildly different fractionation regimens... and eras. What could the benefit from boosting in hypofx be? In Lyon one got 1% improvement from boost, in EORTC about 4%. Think we can get 4% LR improvement when LR risks are ~6% with hypofx without boost? No way. Maybe 2%? On paper, giving 25% more dose and 25% more treatments to 100% of women to help one out of fifty of them (at best) avoid a local recurrence with no improvement in survival sounds crazy. I am not as good as the UK doctors who know exactly who and who not to boost with hypofx (and evidently the boost made no difference but let's not quibble on that), another detail I'm depressingly learning today. I would have to boost all hypofx patients to be sure it was working, because in all the hypofx trials no standard risk factor (grade, age, LVI, not even nodes, etc.) could predict who was at higher risks of recurrence. I couldn't be any better predictor than the trials' data.
Your chart reviews sound pretty reasonable btw.
You have nicely illustrated why we should not blindly boost everyone who walks through the door. The rest is gibberish pretzel logic.
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Who should we boost? A Stage I TNBC patient with a pCR to chemo? Who gets the most benefit from boost in hypofx? Data from hypofx trials, retro- or prospective, would be illuminating.You have nicely illustrated why we should not blindly boost everyone who walks through the door. The rest is gibberish pretzel logic.
"The fact that boost or no–boost had no impact on local recurrence rates [in START] does not mean that boost is worthless. It means that the UK doctors boosted the right patients (the high risk ones) and omitted the boost in the right patients (the low risk ones)." I didn't even call this (not yours) pretzel logic btw...
- Joined
- Feb 8, 2008
- Messages
- 341
- Reaction score
- 485
As is, the evidence for boosting the cavity in a Stage I patient getting hypofx is mighty, mighty weak. Why did this even ever get to be a thing, boosting cavities w/ hypofractionation? Tradition! You can pry standard fractionation from me but you'll take tumor cavity boosts over my dead body. In addition, the patient had a CR to chemo. This puts her form of triple-negative disease, natural history wise, outside typical bad prognosis triple negative disease. Like in the major trials testing hypofx vs standard fx, I never add a boost (because they didn't in the trials, eg START and Whelan). In summary your honor, there is no randomized data to support adding a boost here and extensive evidence that says a pCR is very predictive of improved prognosis (including LR risk) for her. In addition, the reason hypofractionation in breast is associated with slightly better cosmesis is because the total dose delivered was BED equivalent to ~50/25 for α/β ≅ 3 (50*1.67=83, 42.5*1.9=81). This assumption and expectation for equal and/or no worse side effects is completely eliminated if you are a hypofractionator and breast booster.
Scarb, is there literally anything that you do that falls in line with what is generally accepted as normal practice? I have the sense that you just like to debate, but you seem to almost always take a contrarian view. You don't treat nodes, you don't boost...
FWIW Whelan has said himself that he typically boosts, despite the lack of a mandated boost on his trial.
Boost!
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Well heck it's the Internet... and SDN... and I'm a misanthrope. A perfect debate stew.Scarb, is there literally anything that you do that falls in line with what is generally accepted as normal practice? I have the sense that you just like to debate, but you seem to almost always take a contrarian view. You don't treat nodes, you don't boost...
View attachment 272111
FWIW Whelan has said himself that he typically boosts, despite the lack of a mandated boost on his trial.
Boost!
I have seen in my own neck of the woods the local academic center give breast boost to ALL hypofx patients. So which: "blindly" apply to all, or choose judiciously? Upon whom is breast boost in hypofx shown to influence outcome?
If someone ran a trial that established a new standard and publicly announced he "typically" never treats in the manner of the trial, that would "typically" be a news item. AFAIK boosting in hypofx is not "normal practice," nor is routine RNI (especially for N0 patients, of which ~50% made up the EORTC RNI trial e.g., and they kind of derived the biggest benefit from RNI vs N+). I have posted this before but over the last decade only ~4 out of 100 rad oncs worldwide routinely applied RNI to all 3 sites of IMN/sclav/axilla when doing RNI. Yet MA.20--a trial where all 3 RNI sites were treated in ~100% of patients on the RNI arm--is ostensibly the reason RNI is "normal practice" as you say? What use are these Canadian trials if they establish new practices but then no one treats according to the trials, not even the guy who ran the trials*? What is normal? BTW, I never said I don't treat (any) nodes. However, yes, I never boost w/ hypofx. (Non-boosters should be sitting pretty when APM comes down the pike...it's predicted we'll all be treating breast in 5 fractions one day anyways rendering all this brouhaha moot.)
Why say this is "normal practice" (or "I usually look for reasons not to boost but this one ain't it, chief") when there's weak to no data? Or conflicting data in opposite directions. I'm just a real skeptic when people think they have the market cornered on the "right" answer when the data is a big mishmash of low single digit numbers positive and negative sans any effect on natural histories. But yet, this actually describes much of "normal practice" in radiation oncology.
*What Whelan once said about the boost:
In our trial, additional boost radiation to the lumpectomy site was not used, because data from randomized trials supporting its efficacy were not available. Since the completion of this study, results from randomized trials indicate that boost radiation has a modest impact on local recurrence at the expense of cosmetic outcome. In the recently published EORTC trial, the rates of local recurrence at 5 years were 7.3% in the arm receiving breast irradiation alone and 4.3% in the arm receiving the boost radiation. The observed local recurrence rates in our trial were lower: 3.2% in the long arm and 2.8% in the short arm. Although there are limitations to cross-study comparisons, there are some important differences between the two studies. The EORTC trial was conducted in women with negative (78%), positive (21%), or unknown (1%) axillary lymph node status, whereas we enrolled only women with a negative axillary lymph node status. In the EORTC trial, 28% of women received adjuvant systemic therapy, whereas in our trial 52% of women received systemic therapy. In the EORTC study, the benefit of boost radiation appeared mainly in women 50 years of age or younger; in these women, the rate of local recurrence was high—as high as 20% in women who were aged younger than 40 years. In our trial, no difference was detected between groups including younger women; in younger women, the rates of local recurrence were low, especially for women treated with the shorter course of radiation therapy (i.e., 3.6%). For such women, we would postulate that the benefit achieved with boost radiation would be of a relatively small magnitude. Although Bartelink et al. recommend boost treatment for all women who are younger than 50 years old, in our view, this remains an area for future study.
Last edited:
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
I somehow missed (the gist of) your response earlier.I actually kind of agree with scarb on this one. I think we all snap to boosting TNBC beceause of historical outcomes. However, as is well accepted, pCR to NAC in TNBC (I mean really in any non-Luminal A breast cancer) is a huge predictor of improved LRR.
While I would probably still boost with a 39-year old (after discussion of potential toxicities), I don't think it's unreasonable to skip the boost in somebody who has a pCR. Somewhat dependent on patient preferences IMO.
The difference in event-free survival w/ pCR vs no pCR... P≅0.00000000117.
The day an RT trial or meta-analysis of event-free survival shows one part in one billion against the null hypothesis will be a day of rejoicing indeed.
Last edited:
- Joined
- Dec 17, 2007
- Messages
- 3,702
- Reaction score
- 5,209
Or why we should...You have nicely illustrated why we should not blindly boost everyone who walks through the door. The rest is gibberish pretzel logic.
Apart from this, the lack of data is clear in the hypofractionated era. However I would not throw all the data we have out of the window, just because we don't have trials randomizing to boost or no boost in the hypofractionated era. I believe that one would be on the safe side, if one was to boost as the UK doctors did in the START trials. And they most certainly did that according to the data on boost coming from trials on normofractionation.
It's the best evidence we have and it's certainly safer than saying you wouldn't boost anyone.
I could also go on pointing out that in terms of toxicity START did not see any differences between the women boosted or not boosted (although it was not designed to look into that, this was also evaluated). Thus one can make the point that delivering a boost will not cause measurable side effects, at least if the boost dose and technique is similar to that used in START. So, why not to boost?
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
"Or why we should..." I do see, as best as I can tell, that boosters are "class" boosters. They boost everybody, all the time. Maybe I'm wrong 'bout that. I certainly am in normofractionation.I believe that one would be on the safe side, if one was to boost as the UK doctors did in the START trials. And they most certainly did that according to the data on boost coming from trials on normofractionation.
...
I could also go on pointing out that in terms of toxicity START did not see any differences between the women boosted or not boosted (although it was not designed to look into that, this was also evaluated)
...
So, why not to boost?
START
So why not boost, you ask. 1) There is no data from START re: boost criteria; the criteria were undoubtedly hodgepodge and not consistently applied.
Last edited:
- Joined
- Oct 5, 2013
- Messages
- 812
- Reaction score
- 547
You boost everyone all the time with standard frac and yet you talk about ‘cost’ as a reason not to boost in hypofrac?
The logic doesn’t follow at all
Again - you said that 40/15 was biologically the same as 50/25. This isn’t that hard of a concept. If you think a patient will benefit from a boost, that shouldn’t change no matter the initial treatment. There’s no reason that should change with hypofrac
The logic doesn’t follow at all
Again - you said that 40/15 was biologically the same as 50/25. This isn’t that hard of a concept. If you think a patient will benefit from a boost, that shouldn’t change no matter the initial treatment. There’s no reason that should change with hypofrac
- Joined
- Dec 17, 2007
- Messages
- 3,702
- Reaction score
- 5,209
"Or why we should..." I do see, as best as I can tell, that boosters are "class" boosters. They boost everybody, all the time. Maybe I'm wrong 'bout that. I certainly am in normofractionation.
STARTrandomized patients before boost decision was madeboost decisions "needed to be planned before randomisation to ensure that the independent effect of tumour bed boost radiotherapy on adverse effects did not affect the comparisons of fractionation schedules." And there is zero info from START re: criteria for boost. When you say "The UK doctors knew the right patients to boost, and that's why boost wasn't associated with improved LR," how do you really know? It's just a faith-based guess relying on unknown people to apply some unknown criteria to an unknown subset of patients yielding unknown outcomes. Boosting was left up to each individual center's personal preference. Data from normofractionation trials, EORTC etc., like Whelan said, "indicate that boost radiation has a modest impact on local recurrence at the expense of cosmetic outcome." At least we could say that from normofractionation trials; we can't say that from hypofx trials. When START looked at toxicity, there was a non-significant difference in boost/no boost in disfavor of boost. Not surprising, and honestly not especially concerning. But where's the benefit?
So why not boost, you ask. 1) There is no data from START re: boost criteria; the criteria were undoubtedly hodgepodge and not consistently applied.And patients were randomized before boost decision was made.And the HR's for LR for boost/no boost in START were spookily/eerily similar to a degree almost never seen... you might say so similar as if boost didn't matter for LR in hypofx. If you only looked at the START data. Or if you only looked at Whelan. 2) Boost increases cost of tx. 3) Boost increases tx time. 4) The risk(downside)/benefit ratio of boost could be high, simply because the benefit is so small, or unknown. That's the thing: you can't have a cogent discussion of risk/benefit ratio of boost in hypofx. When a benefit is very unknown/questionable, but the cost and convenience and clinical toxicities are not theoretical (and they never are when you use more dose), I tend to demur.
1. I do not boost everyone the whole time. I generally boost more patients than I don't boost. So let's say "I dont boost select patients".
2. From the Start B publication:
Departments were required to have a protocol specifying whether patients who had had breast conserving surgery would receive a boost to the tumour bed, and to use an electron field of appropriate energy to deliver 10 Gy in five daily fractions to the 100% isodose, after initial radiotherapy.
So I presume the sites were supposed to state whether they boost or not and based on what factors they do or do not. It seems that these protocols were very good, since they obviously boosted the right patients... 🙂
- Joined
- Dec 18, 2015
- Messages
- 3,217
- Reaction score
- 4,930
Cost is just one of many reasons. There are two types of “biologically the same as": tumor effects and late effects. If you go back to what I said above... "[42.5/16] was BED equivalent to ~50/25 for α/β ≅ 3 (50*1.67=83, 42.5*1.9=81)"... I never cast a BED aspersion for tumor effects (and never mentioned 40/15). Clinical data always trumps radiobiological theory. Here's what the clinical data has revealed for normofractionation (N), normofractionation plus boost (N+B), hypofractionation (H), and hypofractionation plus boost (H+B). Regimens showing higher tumor BED (ie decreased LR risk) in red; when equal, in green...You boost everyone all the time with standard frac and yet you talk about ‘cost’ as a reason not to boost in hypofrac?
The logic doesn’t follow at all
Again - you said that 40/15 was biologically the same as 50/25. This isn’t that hard of a concept. If you think a patient will benefit from a boost, that shouldn’t change no matter the initial treatment. There’s no reason that should change with hypofrac
1) N vs N+B
2) N vs H
3) H vs H+B, (and H vs N+B, etc.), alternatively (here vs here) H vs H+B (but nothing randomized)
"This isn't that hard of a concept" you say. Holding two competing thoughts in one's mind (in this case that boost does something in normofractionation but does nothing in hypofx) is probably a hard concept.
You have learned well...It seems that these protocols were very good, since they obviously boosted the right patients... 🙂
Last edited:
- Joined
- Apr 21, 2011
- Messages
- 3,744
- Reaction score
- 9,565
The American trial boosted everyone. The Canadian trial boosted no one. The UK trial boost about 50%.
Feel free to boost or not boost whoever you want. Benefit may be minimal, but so is the toxicity. Those 4-5 fractions won't bankrupt the system, especial once bundled.
One exception. Don't "not boost" the lady in the OP. She needs it. Based on a solid footing of randomized data? No.
Feel free to boost or not boost whoever you want. Benefit may be minimal, but so is the toxicity. Those 4-5 fractions won't bankrupt the system, especial once bundled.
One exception. Don't "not boost" the lady in the OP. She needs it. Based on a solid footing of randomized data? No.
Similar threads
- Replies
- 32
- Views
- 3K