Alright so I just need help clearing up this one deal regarding cell potential.
The TBR books say E =Eox + Ered
but some practice problems from the AAMC says E =Ecathode - Eanode
Im just having trouble understanding why the second equation would be minus instead of plus.
thanks.
Reduction occurs at the cathode and oxidation occurs at the anode.
E=Ecathode-Eanode is the same as E=Ereduction/cathode + -Eanode/oxidation... whenever calculating cell voltage the most positive reduction potential (on a table of given reduction potentials) is the reaction that occurs at the cathode..This term always goes "first" then whichever is your 2nd reaction that has a less positive reduction potential, that becomes your oxidation potential. You can directly subtract this potential from the reduction potential OR flip it's reduction potential and add the two...this is saying the same thing (ie. 4-2=2 OR 4+(-2)=2))
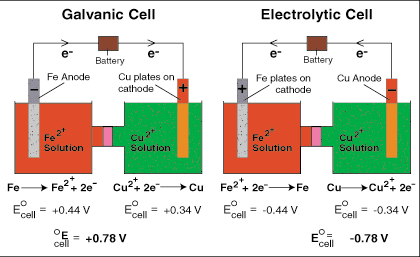
In the above example, take a look at the galvanic cell:
EFe oxidation=+.44V
ECu red= +.34V
METHOD 1
Using equation E=Eox+Ered, the answer is .44 +.34V=
+.78V
However, I could have given you the reduction potentials for both cells:
Fe
2+ + 2e
- ---> Fe -.44V (flipped b/c originally oxidation potential in diagram)
Cu
2+ + 2e
- --> Cu +.34V
Now this is similar to an MCAT question. First, determine which is the more positive potential (the one more likely to be reduced)...in this case it is the Cu2+, the Cu2+ will be reduced and becomes the voltage at the cathode, the Fe2+ has a negative reduction potential and therefore Iron will be oxidized at the anode. If you leave them both as reduction potentials you must apply Method 2:
METHOD 2 (Use when potentials are written as reduction potentials)
E=Ecathode-Eanode
E=E(more positive) - E(less positive)
E=+.34V- (-.44) = +.78V
If you use method 2, leave the negative sign in tact on any negative reduction potentials as this is the whole point in writing it out as Ecathode-Eanode.
Thus, both methods 1 and 2 provide the same result, but are used in different cases...Method 1 is used when you are given the oxidation and reduction potentials separately. Method 2 is used when you are given both of the potentials as reduction potentials.
Because the Ecell is positive (+.78V), the cell is a galvanic cell and spontaneous.