I am trying to count charges and don't get this to add up.
H,R,K each give +1, and D,E each give -1. Is there more to it than this?
I thought option C resulted in +3 -3 = 0, but the solution says it is net -1.
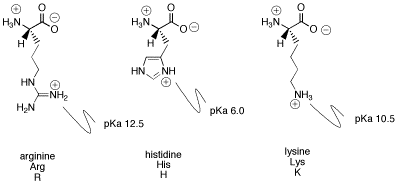
I suspect histidine has a 0 charge at pH = 7. Now
@aldol16 may disagree with the details as to why, but note that the pKa of the histidine side chain is 6.0.
Using the Henderson-Hasselbalch equation, we see the following:
pH = pKa + log([base]/[acid]) -->
pH = pKa + log([deprotonated]/[protonated]) -->
7 = 6 + log([deprotonated]/[protonated]) -->
1 = log([deprotonated]/[protonated]) -->
[deprotonated]/[protonated] = 10
Protonated and deprotonated refer to the state of the histidine side chain. At pH = 7, 91% of histidine concentration is deprotonated and 9% is protonated. To me, 9% is small enough to be negligible and so histidine has approximately a neutral charge at pH = 7.
Based on conversations with aldol16 in a recent past thread, the acidity of histidine depends on its local environment, and the above calculations apply only to histidine in isolation. Histidine located closely with glutamate and aspartate can participate in electrostatic ion pair interactions, and histidine is therefore likely to have a positive charge even at pH = 7.0. However, the question assumes that the peptides listed are all short and linear, and it also assumes that there are no tertiary interactions involved (so no electrostatic interactions). The question wants to simply use the properties of amino acid chemistry to calculate the overall charge of the peptide.
The overall point is that you are right with the peptide in Choice C having a 0 charge because histidine and aspartate at the endpoints of the peptide can simply engage in electrostatic interactions, meaning that histidine does in fact have a +1 charge. I think this question is a poor one that makes too many complicated claims and assumptions when it's better to just let histidine have a +1 charge, since it's more intuitive to approach it that way.