You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
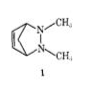
Chiral or achiral?
- Thread starter SaintJude
- Start date
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
I am fairly sure this is a Meso compound.
What is its optically active stereoisomer?
- Joined
- Mar 14, 2007
- Messages
- 118
- Reaction score
- 7
What is its optically active stereoisomer?
Sorry It has been a little while (appx 5yrs since I took my Ochem classes) Why aren't the two carbons opposite each other that are the base of the methyl bridge chiral? They have 4 different substituents.
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
Sorry It has been a little while (appx 5yrs since I took my Ochem classes) Why aren't the two carbons opposite each other that are the base of the methyl bridge chiral? They have 4 different substituents.
You are correct in the general case. The problem with this compound is that you have additional structural restrictions from the bridge with the C atom. Chirality at this points would mean one of the bonds going up and the other away from the ring (where the H currently is). That requires a huge stretch for the C-C bond and cannot happen.
There is another contortion with one bond up, one down and the connecting C in the middle of the ring but that's only a conformational isomer - you can there only with rotations.
In a summary: you need 4 different atoms in the chiral center but you also need to be able to "swap" at least two of them. Sometimes the structure of the molecule will give you no such option.
---
I am here: http://maps.google.com/maps?ll=47.653492,-122.316448
- Joined
- May 27, 2011
- Messages
- 1,710
- Reaction score
- 35
You are correct in the general case. The problem with this compound is that you have additional structural restrictions from the bridge with the C atom. Chirality at this points would mean one of the bonds going up and the other away from the ring (where the H currently is). That requires a huge stretch for the C-C bond and cannot happen.
There is another contortion with one bond up, one down and the connecting C in the middle of the ring but that's only a conformational isomer - you can there only with rotations.
In a summary: you need 4 different atoms in the chiral center but you also need to be able to "swap" at least two of them. Sometimes the structure of the molecule will give you no such option.
---
I am here: http://maps.google.com/maps?ll=47.653492,-122.316448
Say WA? The topmost bridgehead carbon has four different substituents. It is chiral. And, due to molecular symmetry, the molecule is achiral.
If you build this molecule with a kit (yes I still have mine
 ) you realize that like all bicyclic compounds, this one is shaped like a barrel. You could easily switch the nitrogens and the alkene carbons, making the top carbon an S or an R.
) you realize that like all bicyclic compounds, this one is shaped like a barrel. You could easily switch the nitrogens and the alkene carbons, making the top carbon an S or an R.If you replaced the bottom nitrogen with a phosphorus, would you still argue that the topmost bridgehead carbon is achiral? Because then the molecule would certainly be chiral.
Last edited:
- Joined
- Mar 14, 2007
- Messages
- 118
- Reaction score
- 7
Please I am in a fragile state of understanding...what is the answer?
MT Headed, how is the topmost bridgehead carbon chiral? Doesn't it have 2 Hydrogen bonds?
No, if you look closely the carbon is connected to a Nitrogen, a carbon that has 2 hydrogens and is the bridge, and a carbon with one hydrogen in a double bond, as well as a hydrogen (not shown). It has 4 substituents and is 'S' I think. Since the carbon directly across from it (connected to the carbon bridge that is coming out of the page) has the same atomic attachment but different arrangement it has the opposite 'R' configuration.
Since the compound is a mirror image with 2 chiral centers that are opposite configuration, I'd say it is Meso.
- Joined
- May 27, 2011
- Messages
- 1,710
- Reaction score
- 35
No, if you look closely the carbon is connected to a Nitrogen, a carbon that has 2 hydrogens and is the bridge, and a carbon with one hydrogen in a double bond, as well as an hydrogen (not shown). It has 4 substituents and is 'S' I think. Since the carbon directly across from it (connected to the cabron bridge that is coming out of the page) has the same atomic attachment but different arrangement it has the opposite 'R' configuration.
Since the compound is a mirror image with 2 chiral centers that are opposite configuration, I'd say it is Meso.
As drawn, you can't make any conclusions about the S or R of the top carbon. Three substituents are drawn in the plane (which could not possibly be accurate) and the fourth hydrogen is not drawn at all.
In any event, the way you should approach this specific question is:
(1) They are asking if the molecule is chiral,
(2) The molecule has an obvious plane of symmetry, therefore
(*) The molecule is achiral.
The rest of us are just arguing obscure technical points which in a real MCAT exam would be an utter waste of your valuable time. On the MCAT you answer the question being asked, and move on as quickly as possible.
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
Say WA? The topmost bridgehead carbon has four different substituents. It is chiral. And, due to molecular symmetry, the molecule is meso and achiral.
If you build this molecule with a kit (yes I still have mine) you realize that like all bicyclic compounds, this one is shaped like a barrel. You could easily switch the nitrogens and the alkene carbons, making the top carbon an S or an R.
If you replaced the bottom nitrogen with a phosphorus, would you still argue that the topmost bridgehead carbon is achiral? Because then the molecule would certainly be chiral.
Ok, they are chiral centers. What I'm trying to get to is that you cannot change them independently of each other. In other words, if the compound is R,S you cannot make R,R or S,S - your only options are R,S or S,R.
If you had one phosphorus instead of one of the nitrogens R,S and S,R the molecule would not be symmetrical and you would have two enantiomers.
For the case with two nitrogens the molecule symmetry gives you exactly one compound. If you want to call that a meso compound or not is mostly a matter of definition. With the lack of any other stereoisomers I don't see much practical value in designating it as a meso compound.
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
The rest of us are just arguing obscure technical points which in a real MCAT exam would be an utter waste of your valuable time. On the MCAT you answer the question being asked, and move on as quickly as possible.
That. Besides the the obvious point that the molecule is achiral, the argument about it being a meso compound or not is mostly philosophical. Asking it as a question on any exam would be just poor test writing, at least in my opinion.
Last edited:
Ok, they are chiral centers. What I'm trying to get to is that you cannot change them independently of each other. In other words, if the compound is R,S you cannot make R,R or S,S - your only options are R,S or S,R.
If you had one phosphorus instead of one of the nitrogens R,S and S,R the molecule would not be symmetrical and you would have two enantiomers.
For the case with two nitrogens the molecule symmetry gives you exactly one compound. If you want to call that a meso compound or not is mostly a matter of definition. With the lack of any other stereoisomers I don't see much practical value in designating it as a meso compound.
i think that's exactly why it is meso and why this can be figured without having to know its stereochemistry.
- Joined
- Mar 14, 2007
- Messages
- 118
- Reaction score
- 7
As drawn, you can't make any conclusions about the S or R of the top carbon. Three substituents are drawn in the plane (which could not possibly be accurate) and the fourth hydrogen is not drawn at all.
In any event, the way you should approach this specific question is:
(1) They are asking if the molecule is chiral,
(2) The molecule has an obvious plane of symmetry, therefore
(*) The molecule is achiral.
The rest of us are just arguing obscure technical points which in a real MCAT exam would be an utter waste of your valuable time. On the MCAT you answer the question being asked, and move on as quickly as possible.
Ahhh I see your point. It has been a while from Ochem for me. I forgot that even though it has 2 chiral centers it is still considered Achiral. I assumed it was chiral but not optically active and forgot that they are connected unless there is a racemic mixture of enantiomers. Also when I look at it the bridge appears to come out of the screen and I have trouble seeing it in reverse.
indeed. 2 chiral centers. achiral and meso, not optically active.
- Joined
- May 27, 2011
- Messages
- 1,710
- Reaction score
- 35
That. Besides the the obvious point that the molecule is achiral, the argument about it being a meso compound or not is mostly philosophical. Asking it as a question on any exam would be just poor test writing, at least in my opinion.
I stand corrected.
 This molecule is not meso, because it has no stereoisomers. A meso compound requires the presence of optically active stereoisomers, and this molecule has none. It is, of course, achiral as we have all proven. Repeatedly.
This molecule is not meso, because it has no stereoisomers. A meso compound requires the presence of optically active stereoisomers, and this molecule has none. It is, of course, achiral as we have all proven. Repeatedly.Learn something new every day.
Ref: http://goldbook.iupac.org/M03839.html
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
I stand corrected.This molecule is not meso, because it has no stereoisomers. A meso compound requires the presence of optically active stereoisomers, and this molecule has none. It is, of course, achiral as we have all proven. Repeatedly.
Learn something new every day.
Ref: http://goldbook.iupac.org/M03839.html
Nothing to brighten my day like IUPAC agreeing with me.
If you want to be extra mean, you can replace one of the H atoms on the middle C on the bridge with a Br and you'll get yourself a pair of enantiomers which both happen to be meso compounds.
i don't understand. why doesn't it have stereocenters? the carbon has 4 different substituents, does it not?
how does it differ from something like
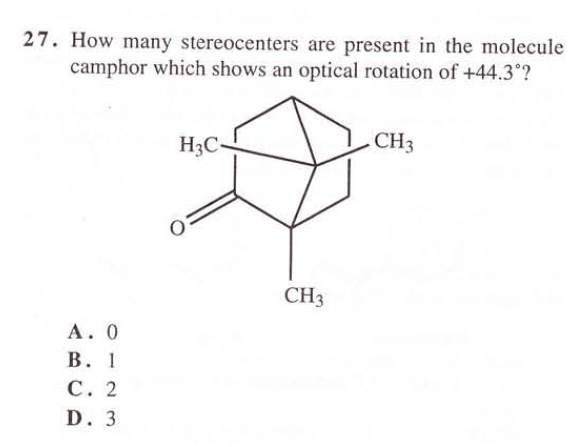
where both top and bottom most carbons are stereocenters? (answer is C)
how does it differ from something like

where both top and bottom most carbons are stereocenters? (answer is C)
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
i don't understand. why doesn't it have stereocenters? the carbon has 4 different substituents, does it not?
how does it differ from something like

where both top and bottom most carbons are stereocenters? (answer is C)
We agreed that it has two stereo centers. But because you cannot change one without changing the other, you cannot make all the theoretical stereoisomers. So you end up with only two and they happen to be the same molecule.
If you look the IUPAC definition which MT Headed posted, to have a meso compound you need at least one optically active stereoisomer. Since our initial compound is achiral and has not other stereoisomers, it does not make the cut for being a meso compound.
Adding another stereocenter somewhere (like middle bridge carbon or extending the methyl groups) has the potential to make a set of optically active isomers and will be a meso compound.
- Joined
- May 27, 2011
- Messages
- 1,710
- Reaction score
- 35
If you look the IUPAC definition which MT Headed posted, to have a meso compound you need at least one optically active stereoisomer.
True...
If you want to be extra mean, you can replace one of the H atoms on the middle C on the bridge with a Br and you'll get yourself a pair of enantiomers which both happen to be meso compounds.
Well, they would both be diastereomers of each other, and therefore they belong to a set of diastereomers, but none of the members of this set are optically active. Therefore they still do not meet the IUPAC definition of meso compounds.
Of course, there is no way in hell the MCAT would test this kind of esoteric knowledge.
Last edited:
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
True...
Well, they would both be enantiomers of each other, and therefore they belong to a set of diastereomers, but none of the members of this set are optically active. Therefore they still do not meet the IUPAC definition of meso compounds.
Of course, there is no way in hell the MCAT would test this kind of esoteric knowledge.
Now that stopped making sense. I always considered optically active to be equivalent of being chiral. And I'm fairly certain that making the mid-bridge carbon chiral makes the whole molecule chiral.
On the other hand, we still have a plane of symmetry - the middle of the 'barel', parallel to the lid/bottom. Which should make it achiral. But I cannot picture how you can superpose the mirror image on top of the original.
Edit: Never mind, of course you can superpose the mirror image. That makes them two diastereomers, none of them being an enantiomer. An enantiomer for multichiralcenter molecule needs to have all the chiral centers flipped, not just one.
Last edited:
- Joined
- Dec 30, 2009
- Messages
- 2,641
- Reaction score
- 527
Oh! So it does have stereoisomers, but it is achiral and it is not meso because it must contain possible chiral configurations as well.
Yes. The original question was only chiral vs. achiral. So the rest is really a beer conversation without the beer.
Now that stopped making sense. I always considered optically active to be equivalent of being chiral. And I'm fairly certain that making the mid-bridge carbon chiral makes the whole molecule chiral.
On the other hand, we still have a plane of symmetry - the middle of the 'barel', parallel to the lid/bottom. Which should make it achiral. But I cannot picture how you can superpose the mirror image on top of the original.
Edit: Never mind, of course you can superpose the mirror image. That makes them two diastereomers, none of them being an enantiomer. An enantiomer for multichiralcenter molecule needs to have all the chiral centers flipped, not just one.
Right. Plane of symmetry = superimposable mirror image even when it isn't readily apparent without the molecular model kit.
Yes. The original question was only chiral vs. achiral. So the rest is really a beer conversation without the beer.
I don't know about you, but I needed a beer to make it through all these explanations!
Similar threads
- Replies
- 5
- Views
- 1K
- Replies
- 4
- Views
- 5K
- Question
- Replies
- 1
- Views
- 6K