- Joined
- Jun 15, 2011
- Messages
- 91
- Reaction score
- 0
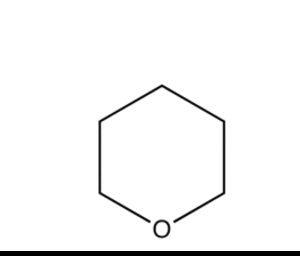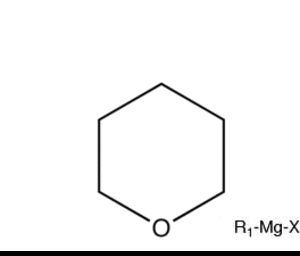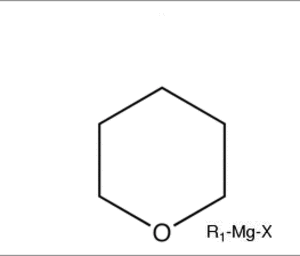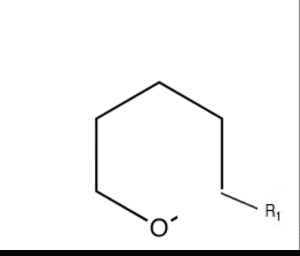
What exactly cuts cyclic ethers or cyclic compounds altogether.
On question #41 on the 2011 DAT destroyer (Orgo section)
It says that a cyclic ether can be cut by grignard reagent. I dont understand how this is possible if Grignard is a base....
On question #41 on the 2011 DAT destroyer (Orgo section)
It says that a cyclic ether can be cut by grignard reagent. I dont understand how this is possible if Grignard is a base....