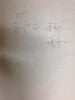- Joined
- Aug 2, 2010
- Messages
- 46
- Reaction score
- 1
Hey guys,
I'm having a really hard time determining how these people determined what was a wedge or dash in the alkene before the reaction happened and after the reaction happened. If you see the image below, it shows the alkene substituents in various wedge or dash notation after the reaction has happened but I have no idea how they made that determination, can anyone help me out?

I'm having a really hard time determining how these people determined what was a wedge or dash in the alkene before the reaction happened and after the reaction happened. If you see the image below, it shows the alkene substituents in various wedge or dash notation after the reaction has happened but I have no idea how they made that determination, can anyone help me out?