In the science workbook there's a question that asks "Which of the following molecules have a dipole moment" and the answer is that CCl3H has a dipole moment, which it does.
But there is also a cis alkene. wouldnt a cis alkene have a dipole moment as well?
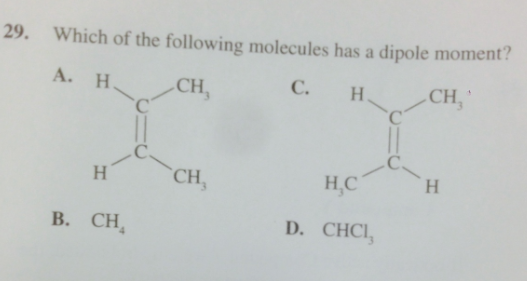
But there is also a cis alkene. wouldnt a cis alkene have a dipole moment as well?
