- Joined
- Feb 27, 2016
- Messages
- 59
- Reaction score
- 38
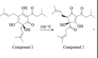
Hi! Confused about #21 EK FL2 C/P -
Does intramolecular hydrogen bonding affect the polarity of a total molecule?
In the problem, there are two compounds. Compound 1 has a hydroxyl and carbonyl oxygen (from ketone) adjacent to each other. Compound 2 has two carbonyl oxygens (from ketones) adjacent to each other. The question wants to know which will elute first with the polar mobile phase. I know alcohol groups are more polar than ketones, but does intramolecular H bonding with the -OH and =O affect polarity at all?
Does intramolecular hydrogen bonding affect the polarity of a total molecule?
In the problem, there are two compounds. Compound 1 has a hydroxyl and carbonyl oxygen (from ketone) adjacent to each other. Compound 2 has two carbonyl oxygens (from ketones) adjacent to each other. The question wants to know which will elute first with the polar mobile phase. I know alcohol groups are more polar than ketones, but does intramolecular H bonding with the -OH and =O affect polarity at all?
Attachments
Last edited: