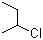
EK is saying that (S)-sec-butyl-chloride will react with NaOH in a Sn2 reaction and therefore the carbon will have inversion of configuration. I believe tertiary alkyl halides will almost always go Sn1 because of the stability of the carbocation. Any ideas?
FYI in EK (2007) orgo, they specifically say that tertiary substrates will not undergo Sn2. So I'm guessing this is errata, oh well.
FYI in EK (2007) orgo, they specifically say that tertiary substrates will not undergo Sn2. So I'm guessing this is errata, oh well.