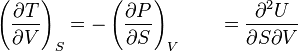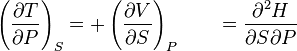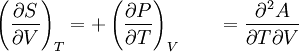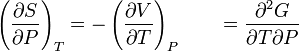So I just can't seem to get these down. I have studied and studied and I am still missing them in the 1001 chem book. Is there an easy way to understand these? Do I just need to memorize the info? I get confused when the question starts throwing out changes in systems, surroundings, universe and says as one inc. one dec. and this is the outcome, etc.
How are you getting this info? Is this big on the mcat?
Dear CarolinaGirl,
I apologize on behalf of my really smart peers for throwing on you details usually taken into consideration only in Pchem and Thermo classes. There are actually just a few things that you will need to know for the MCAT, they are all covered in your local Freshman Chem textbook, very uninterestingly, that is. The following should serve you as an introduction.
Systems are part of the universe that we are interested in. You (or MCAT) choses the system. Nothing is special about it.
Isolated systems cannot exchange matter or energy with their environments. (an insulator)
Open systems are the opposite.
Closed systems can exchange heat but not matter.
1. Thermodynamic questions are common in the MCAT because they really affect all science. You should know that the
First Law is that Energy is conserved. No surprise here. If someone proves this wrong, scientists are out of business.
The first law also says that in most situations, energy (U) comes in the form of either work (w) or heat (q). U = W + Q. So if you got a system that has a certain amount of internal energy, you won't just lose this energy since it is conserved. The only way that the energy of that system can be decreased is by heat or work. You may know in physics that work is w= F*d = P*V. Or you know now. Heat is still not defined, we think that we know what it is, but we don't.
2.
The Second Law introduces Entropy. What it says is that
all processes in this world that happen spontaneously will result an increase in entropy, or at the very least, the entropy should remain the same. You can think of entropy as a measure of disorder, but it is defined mathematically as
dS = dq/T, where T is the absolute temperature. Entropy is a very powerful concept.
3.
The third law of thermodynamic makes the bold assumption that
we cannot reach absolute zero. At the limit of that temperature, entropy of all system is zero. However, it does not mean that all molecules would become static (no type of motion whatsoever) at 0 K.
Yes, I knew that all you asked me for was enthalpy and entropy, but you need to understand the above concepts before going futher. Good.
Now that we have talked somehow about entropy, let's talk about enthalpy.
Enthalpy is just a mathematical expression defined as H = U + PV. The only reason that this ENTHALPY thing was invented is because the change in enthalpy turns out to be equal to the energy of a system when pressure is constant. The advatage is that the CHANGE in enthalpy is easily measurable. For experimental scientists, it means that it is worth going through the headache of confusing generations of science students.
If you have an enthalpy problem, nine out of ten times, all you will have to do is take the change in enthalpy of the products and substract from it the change in enthalpy of the reactants. You should multiply everything by their stoichiometric coefficients, that means that you usually need a balanced equation. Know that a reaction with negative DH is exothermic and gives off heat, while positive DH is endothermic and absorbs heat. This might not be intuitive. Make sure you know it.
Seriously, I just answered all your questions. But there are related questions that MCAT wants you to know about.
There is a concept called Gibb's Free Energy, (DG) it supposed to mean the amount of useful work that can be obtained from a system at constant temperature and constant pressure. The famous MCAT equation is
DG = DH - TDS. DG has to be negative for a reaction to be spontaneous. A -DG is
exerGONIC, a +DG is
endergonic. This equation implies that just because a system is exoTHERMIC, does not mean that it will be spontaneous.
I think that you are set.
Now some demystifications from previous posts:
- You need to learn definitions for certain questions
Adiabatic: no heat in or out
Isothermal: temperature is constant
Isobaric: pressure is constant
Isochoric:constant volume
- And a few facts
- Heat taken by no system while there is no phase change:
q=mcDT (mcat!)
- For gases, U is only dependent on temperature, so
DU is 0 for isothermal processes. Then from first law, 0= Q+W or W=-Q. (and its not wrong for ideal gases. Don't know about other things.)
- For an
adiabatic process, no heat is transfered so, U = 0 + W.
Anyone needs a science tutor?