"Ionic liquids are made of large organic cations and inorganic anions and exist in the liquid phase at room temperature. Even weakly basic anions can deprotonate substrates in these highly concentrated ionic liquids, causing unwanted side reactions. Which anion would be the least likely to produce unwanted side reactions in an ionic liquid?
a) BF4-
B) AlCl4-
C) SO42-
D) SbF6-
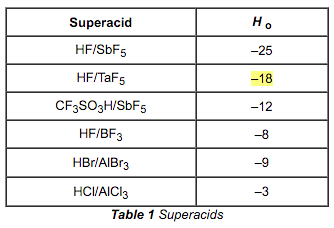
(H0 = Hammett function = a measure of acidity that is used for very concentrated solutions of strong acids, including superacids)
The explanation is:
D. The question is asking for the weakest base. The weakest basic anion will be the conjugate of the strongest acid. Since the answer will come from Table 1 and the question is asking for an extreme, the middle value of the variable in choice A can be eliminated. All superacids are stronger than pure H2SO4, eliminating choice C. SbF6– is the conjugate of SbF5/HF, which is the strongest acid because it has the lowest Ho value. Therefore, choice B is eliminated and choice D is the correct answer.
Why does it say, "The question is asking for the weakest base"?
The question stem asks, "Which anion would be the least likely to produce unwanted side reactions in an ionic liquid?"
It also says that "weakly basic anions can deprotonate substrates...causing unwanted side reactions."
Wouldn't you want to avoid a weak base (and thus strongest acid) if you're trying to avoid unwanted side reactions?
Admittedly I'm weak on acid/bases. Can anyone explain this to me? Thank you!
a) BF4-
B) AlCl4-
C) SO42-
D) SbF6-
(H0 = Hammett function = a measure of acidity that is used for very concentrated solutions of strong acids, including superacids)
The explanation is:
D. The question is asking for the weakest base. The weakest basic anion will be the conjugate of the strongest acid. Since the answer will come from Table 1 and the question is asking for an extreme, the middle value of the variable in choice A can be eliminated. All superacids are stronger than pure H2SO4, eliminating choice C. SbF6– is the conjugate of SbF5/HF, which is the strongest acid because it has the lowest Ho value. Therefore, choice B is eliminated and choice D is the correct answer.
Why does it say, "The question is asking for the weakest base"?
The question stem asks, "Which anion would be the least likely to produce unwanted side reactions in an ionic liquid?"
It also says that "weakly basic anions can deprotonate substrates...causing unwanted side reactions."
Wouldn't you want to avoid a weak base (and thus strongest acid) if you're trying to avoid unwanted side reactions?
Admittedly I'm weak on acid/bases. Can anyone explain this to me? Thank you!
