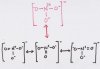A sample DAT question asked how many ground-state resonance structures there are for NO3-. I can draw 4 structures, but the correct answer listed is 3. Why is the structure in red not valid? It still has an overall charge of -1. All resonance structures do not have to be equal energy. What do they mean by ground-state resonance structures? I can't even find the concept in my chemistry textbook.
Ground State Resonance Structure
- Thread starter UBDent19
- Start date