- Joined
- Jul 6, 2014
- Messages
- 1,535
- Reaction score
- 1,822
I'm not sure if this makes sense but I'll try to explain it the best I can:
This has to do with finding the H-NMR of a proton: Lets say there's 1 proton on each side of a proton we're trying to find the peak of, one of the adjacent protons is in R conformation and the other in S. Would the H-NMR display the peak as a triplet (if the protons are considered symmetrical) or would it display as a quadruplet (if the protons are considered different)?
I guess an example molecule would be: (1R-3S)-1,3 dibromo-2-chloro-1,3- dimethylpropane (below) and we're trying to find the peak of the hydrogen geminal to the chlorine.
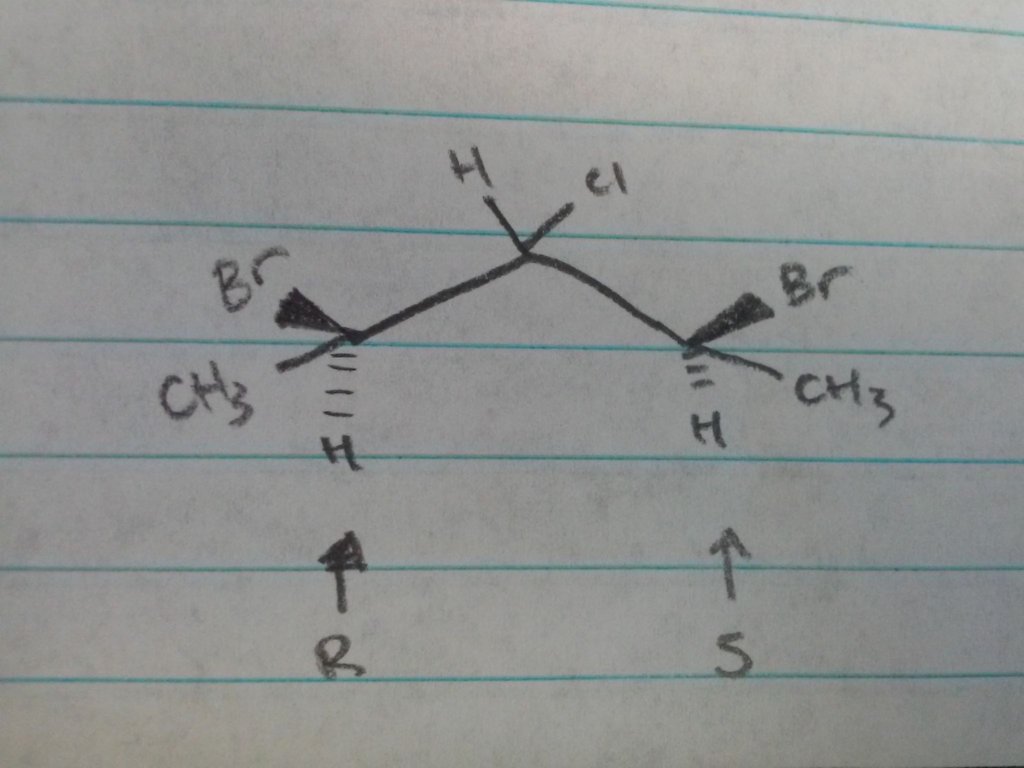
This has to do with finding the H-NMR of a proton: Lets say there's 1 proton on each side of a proton we're trying to find the peak of, one of the adjacent protons is in R conformation and the other in S. Would the H-NMR display the peak as a triplet (if the protons are considered symmetrical) or would it display as a quadruplet (if the protons are considered different)?
I guess an example molecule would be: (1R-3S)-1,3 dibromo-2-chloro-1,3- dimethylpropane (below) and we're trying to find the peak of the hydrogen geminal to the chlorine.
Last edited:
