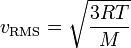Hey guys, I need a little bit of help on understanding diffusion rates.
I know that diffusion rate increases with:
1.) increased velocity of the molecules
2.) increased concentration difference between the two areas
3.) decreased total concentration/ Increase in volume (increased mean free path).
I was wondering what happened as diffusion begins to progress.
--> Does the diffusion rate increase? Because there is less concentration, and hence a greatly increased mean free path.
--> Or does the diffusion rate decrease? Because there is decreased concentration difference.
Also, just wanted to confirm that concentration difference has no effect on Effusion?
I know that diffusion rate increases with:
1.) increased velocity of the molecules
2.) increased concentration difference between the two areas
3.) decreased total concentration/ Increase in volume (increased mean free path).
I was wondering what happened as diffusion begins to progress.
--> Does the diffusion rate increase? Because there is less concentration, and hence a greatly increased mean free path.
--> Or does the diffusion rate decrease? Because there is decreased concentration difference.
Also, just wanted to confirm that concentration difference has no effect on Effusion?
Last edited: