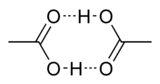
Can the oxygen of a carbonyl group form hydrogen bonds?
The question asks which has most similar boiling point to CH3COOH
Between the answer choices CH3COH v. CH2ClCH2OH...answer is the second one but the first is more similar in molecular weight by a little.
the carbonyl oxygen can function as a hydrogen-bond
acceptor. it cannot function as a hydrogen bond
donor.
acetic acid (CH3COOH) can form hydrogen bonds with itself because the carbonyl oxygen (and the other oxygen) can function as a hydrogen bond acceptor, and the O-H bond functions as a hydrogen bond donor so hydrogen bonding plays an important role in the determination of the boiling point of acetic acid
acetyaldehyde (CH3COH) is an aldehyde, not an alcohol. Just because it says OH doesn't mean it's an alcohol (actually, i think you noticed that based on your question, but I just wanted to emphasize that). acetaldehyde cannot form hydrogen bonds with itself (well, with other molecules of itself, i.e. dimerization) because it can only function as a hydrogen bond acceptor. if you were to put it in water, it could hydrogen bond with water (which is why it's water soluble), but it cannot hydrogen bond with another acetaldehyde molecule.
2-chloroethanol can hydrogen-bond with other molecules of 2-chloroethanol because the oxygen can function as a hydrogen-bond acceptor and the O-H bond can function as a hydrogen-bond donor.
that's why the boiling points of 2-chloroethanol and acetic acid are reasonably close.
so remember, for a pure substance to exhibit hydrogen-bonding, it must be able to function as both a hydrogen-bond donor and as a hydrogen bond acceptor.