FONClBrISCH or FOClNBrISCH
Saw a post from 2009 saying "FONClBrISCH" was incorrect from the TBR books. Kinda confused now for remembering the electronegativity of these common elements.
Or are the electronegativity values for Cl and N just too similar, and so it doesn't really matter?
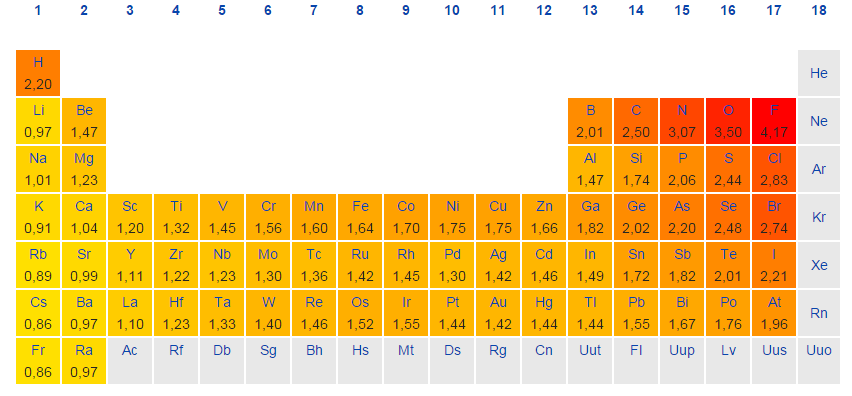

Saw a post from 2009 saying "FONClBrISCH" was incorrect from the TBR books. Kinda confused now for remembering the electronegativity of these common elements.
Or are the electronegativity values for Cl and N just too similar, and so it doesn't really matter?


