- Joined
- Jul 15, 2008
- Messages
- 74
- Reaction score
- 0
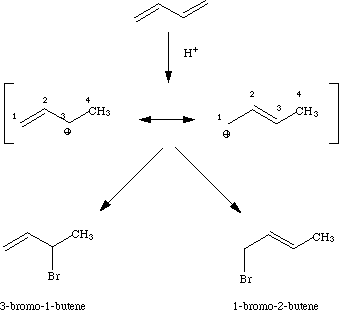
What are the hard and fast rules we should know for the kinetically and thermodynamically controlled rxns in ochem when we've got conjugated structures and we're adding halogens? I'm looking at destroyer #'s 56 &66 and trying to make sense of it...I have no recollection. If I had to guess I'd say kinetic control adds to more stable structures than thermodynamic which adds faster and is prob more influenced by sterics?
Xtian
Xtian