- Joined
- Jul 16, 2003
- Messages
- 6,441
- Reaction score
- 4,782
- Points
- 6,481
- Attending Physician
neochord?@sevoflurane very nice- that is exactly the paper I was referring to. This patient had a basal inferior aneurysm, which I tried to demonstrate in the third image. From the paper: “Our previous analysis did not corroborate these observations and identified only the presence of a basal aneurysm or dyskinesis as an independent predictor of recurrent mitral regurgitation.”
With respect to the thing beneath the mitral valve... Not a clot. Would it change anything if I told you that this surgeon uses a chordal sparing technique for MVR?
Also @anbuitachi no worries dude, there’s a reason you need an extra year of training to do cardiac. If you asked me to discuss spinal cord stims or take care of a sick neonate, I’d be lost
I am guessing not.. likely tissue from mvr chordae sparring w/ tethering. Cool echo. 3D pix polly beautiful. Question is what did you guys do? convert to bioproathetic?neochord?
When this surgeon does MV replacement, he tries to preserve as much of the subvalvular apparatus as possible (I believe there are some benefits in terms of LV geometry and function). He does this by “tucking” the remaining chordal tissue up underneath the ring of the valve. In this case there was a small piece of the head of the anterolateral pap muscles which was part of that chordal tissue, which must have came loose during the process of tying down the valve. It was flopping around in the LV near the LVOT. We showed the surgeon who assured us that it wasn’t going to go anywhere or cause any problems, and we left it alone. I did make it a point to take lots of pictures and comment on it in the echo report so that subsequent echocardiographers don’t see it and freak out, thinking it’s a veg or thrombus.
Anyway, that’s all the cases I’ve got for now- someone else’s turn! 😎
this all day
Also [USER=229930]@anbuitachi no worries dude, there’s a reason you need an extra year of training to do cardiac. If you asked me to discuss spinal cord stims or take care of a sick neonate, I’d be lost
72 y/o for TAVR:
Thoughts?
72 y/o for TAVR:
Thoughts?
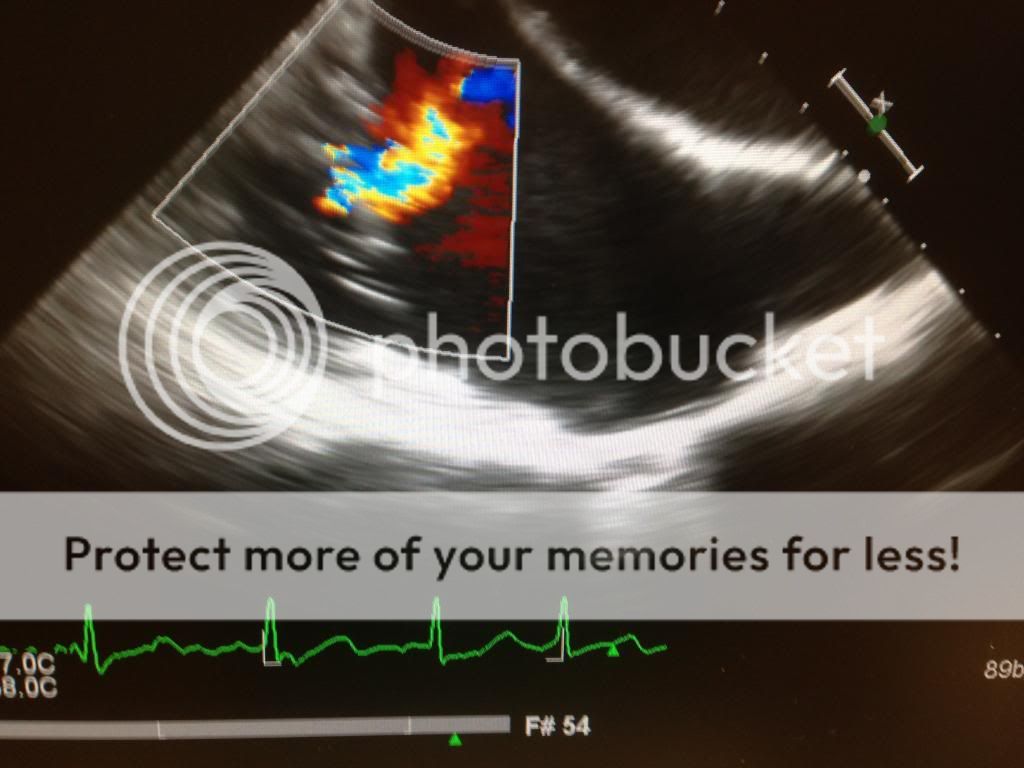
Looks like a papillary fibroelastoma but it's a bit irregular that it'd be on the atrial side
Good question.If you do a TAVI with that on the mitral, risk of causing it to embolize?
I’m surprised that there’s not more MR, based on the 2D it looked like there was malcoaptation - either due to AL leaflet flail or PL restriction. Also appears to be a fair amount of annular calcification, which isn’t surprising... The thought of a mobile chunk of calcium crossed my mind, but I don’t see enough shadowing under the mobile echo density for that the be the case.
Differential otherwise includes papillary elastoma, thrombus, or vegetation. LA myxomata can also very rarely be found on the MV too, if I’m not mistaken. However I think it becomes a bit of a moot point, since whatever it is, the mobility and size seem to suggest that it needs to come out (stroke risk otherwise too high for a 72 yo, assuming he or she is robust enough to tolerate a pump run). I would argue that if you’re going on CPB anyway, don’t dick around with the TAVR and just do the SAVR on bypass.
If you wanted to do the TAVR for whatever reason (let’s say we decided not to go after the thing on the MV), I don’t see how the risk of embolization would be crazy. Granted it’s not nothing, but generally no wires or catheters end up in the LA during a TAVR. Only risk I can think of for causing the thing to flick off during a TAVR would be rapid pacing, or if wires/catheters tug on subvalvular apparatus and cause unusual leaflet motion. Wonder if a Sentinel device would help catch it when it flies off...
Great case
@sevoflurane just curious, what made you think that this was mobile calcium as opposed to myxoma/elastoma/etc?
Ooo that’s a big’un! Must have been fairly symptomatic?
I may have missed this (didn't read the whole thread), but did anyone consider using a Sentinel device during the discussion?Nice synopsis and differential @Hork Bajir . The big question of the case is to cancel or to proceed right? Everyone here is super smart and I get the feeling that nobody thinks that this mass is neither a vegetation or a thrombus which would lead us down the pathway of cancelling the case.
CFD was certainly non-impressive and reassuring as others mentioned above. Took an en face 3D clip to help in decision making. I feel that this is a rare presentation of a large calcium deposit near the posteromedial commissure and you make a valid point with the lack of shadowing. Although mobile (bad sign), I think the only reason it is mobile is because it is attached to A3 which you can clearly see in the 2d x-plane clip/3d en face. Finally, since we are doing a TAVR, we are not doing a transeptal puncture which would give me some reservation.
We decided to proceed and landed a nominal 29 mm Sapien 3 w/o any issues.
Documented a final 3D clip at the end of the case to show it did not embolize.
Nice job everyone.
I may have missed this (didn't read the whole thread), but did anyone consider using a Sentinel device during the discussion?











So it seems they fixed the paravalvular leak but now that MR needs to be addressed. It seems to be a chronic problem because the LA looks dilated with high LA pressure. I'm curious about the mitral pre-bypass although maybe its secondary to that poor LV function.another case. all images post-CPB s/p AVR after pt was told TAVR was not feasible based on TAVR protocol CTA findings.







ECMO venous cannula?some clips




Proteks are placed R IJ like a swan. This device is seen appearing from the IVC...Terrible and dilated RV, some sort of drainage cannula in the RA. Suggestion of significant TR but not well shown. Protek? Vortex? Arguably there is a McConnel’s sign with sparing of the RV apex
Terrible and dilated RV, some sort of drainage cannula in the RA. Suggestion of significant TR but not well shown. Protek? Vortex? Arguably there is a McConnel’s sign with sparing of the RV apex
This is an impella RP with inflow at the IVC/RA junction and outflow in the PA. Little bit tricky in the bicaval because you can't see the cannula continuing into the TV. This pt had severe post-op RV dysfunction coming off pump after AVR/CABG and required MCS. Impella was able to d/c'ed after 3 days and dobutamine continued.ECMO venous cannula?
Yeah that is pretty interesting. I know an LV impella is 3.5-4 centimeters past the AV annulus so I wonder if that's the same distance past the triscuspid annulus?Oh interesting, I haven’t seen any RPellas in person. They were using them at my current institution briefly, but stopped shortly before I begin fellowship because the mortality was upwards of 80%. Now it’s Protek, ECMO, or surgical Centrimag RVAD. What do you look for on echo to confirm proper positioning?
Oh interesting, I haven’t seen any RPellas in person. They were using them at my current institution briefly, but stopped shortly before I begin fellowship because the mortality was upwards of 80%. Now it’s Protek, ECMO, or surgical Centrimag RVAD. What do you look for on echo to confirm proper positioning?
It's gotta be placed using fluoro w/wo TEE imo. Some folks you can pick up a really good ME Asc Ao SAX showing the main and R PA and some you can't. This lady didn't have great windows or else I would've included a shot of the impella pigtail at distal main PA or very proximal R or L PA which is where it should be terminating.Yeah that is pretty interesting. I know an LV impella is 3.5-4 centimeters past the AV annulus so I wonder if that's the same distance past the triscuspid annulus?
oh ok....distal to the pulmonic valve. brain spasm. that makes 100% more senseIt's gotta be placed using fluoro w/wo TEE imo. Some folks you can pick up a really good ME AV SAX showing the main and R PA and some you can't. This lady didn't have great windows or else I would've included a shot of the impella pigtail at distal main PA or very proximal R or L PA which is where it should be terminating.
I think for L sided impella, it depends on the type. For the CP and 5.0, both of which I believe have a pigtail, optimal distance is ~3.5cm past the AV. For the 5.5, sans pigtail, manufacturers recommend 5-6cm beyond the valveYeah that is pretty interesting. I know an LV impella is 3.5-4 centimeters past the AV annulus so I wonder if that's the same distance past the triscuspid annulus?
RV is of paramount importance. If you can't get blood to the LV (via RV ejection), then the LVAD will not work. When you start adding 1-2 l/m to the systemic circulation, that is 1-2 l/m extra flow that the RV suddenly is confronted with. It doesn't have a lot of time to accommodate for such a change in hemodynamics. Therefore, inhaled vasodilators and RV augmentation with volume and inotropes may be necessary. If these maneuvers don't work this may cause the RV to go into failure. One thing to keep in mind is that the RCA (supplies the RV) is anterior. Air tends to go down the RCA when coming off bypass. Furthermore, CPB doesn't protect the RV nearly as well as the LV. This only adds insult to injury when dealing with LVAD placement. Many of the the RVADS that are placed in practice are actually due to an LVAD that overwhelmed the RV leading to RV failure.
The "suckdown" phenomena is usually due to an underfiled LV causing the interventricular septum to get pulled towards the LV free wall. Doing this alters the geometry of the RV. Augmenting the geometry of the RV may equate to poor systolic performance AND tricuspid annulus dilatation leading to acute tricuspid regurgitation.
Here is a modified bicaval view showing this:
If you see this coming off bypass you need to think: oh... oh... what can we do to make this better.
The answer...? As weird as it sounds, reduce your flow rates on your LVAD and augment volume status. These maneuvers will return the RV to it's regular shape and hopefully increase RV unloading to the LV.
In addition to causing the suckdown phenomena, the inflow canulas can become obstructed. Check for this by using color Doppler and seeing laminar flow. You can also use CWD. Flows should be less than 2.5 m/s. Similarly, the outflow cannula (ascending aorta) should show slightly lower flows. 1-2 m/s.
AI is bad... LVAD worsens AI and therefore you may not achieve increased organ perfusion. You can create a partial closed loop from the ascending aorta back into the LV causing a volume overload problem. You need a competent valve. What are the choices?
1) Repair the valve.
2) Replace the valve and deal with higher morbidity/mortality. Never use mechanical valves. Always bioprosthetic ones.
3) Sounds crazy... but if it's destination therapy or bridge to transplant, you can surgically sew the aortic valve shut so that it doesn't open at all! 😱
Good stuff.
Lol I excitedly clicked on this bumped thread hoping you wouldAnybody have interesting echos they have had recently?
Lol I excitedly clicked on this bumped thread hoping you would
A few good Mitral Clip cases earlier this week. I might dig ‘em up and post them.
Here is today’s case. Nothing crazy.
Anyone want to guess what is happening here?