- Joined
- Jul 12, 2015
- Messages
- 26
- Reaction score
- 4
Hello all,
First off, happy holidays!
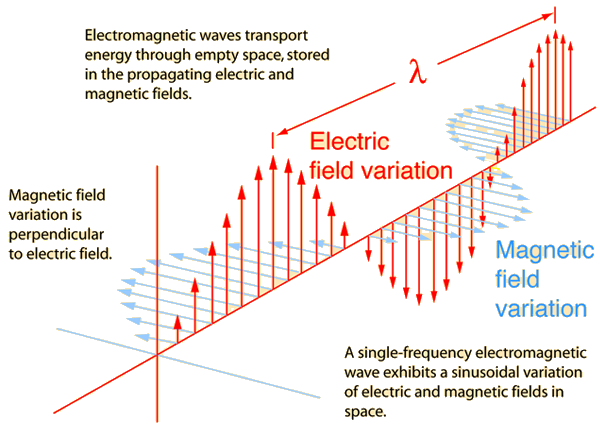
I'm having some trouble visualizing what electromagnetic radiation is. If all electrons are constantly vibrating around the nucleus and have rotational acceleration, is it correct to say that electrons are constantly producing changing electric and magnetic fields? Is this not the definition of electromagnetic radiation? However, light is only emitted when electrons fall from an excited energy state to a ground state. What is the difference between this emitted light and electromagnetic radiation produced from electrons simply vibrating about? Is light electromagnetic wave that has detached from the source?
Thank you for any help.
First off, happy holidays!
I'm having some trouble visualizing what electromagnetic radiation is. If all electrons are constantly vibrating around the nucleus and have rotational acceleration, is it correct to say that electrons are constantly producing changing electric and magnetic fields? Is this not the definition of electromagnetic radiation? However, light is only emitted when electrons fall from an excited energy state to a ground state. What is the difference between this emitted light and electromagnetic radiation produced from electrons simply vibrating about? Is light electromagnetic wave that has detached from the source?
Thank you for any help.