- Joined
- Sep 12, 2011
- Messages
- 431
- Reaction score
- 34
Besides, just memorizing the VSEPR chart of shapes and angles, does anyone have a useful or intuitive way of remembering all this information? 





There was a question on my mcat a few years ago, asking about the molecular shape.
The answer was apparently square planar, a shape i never even heard of before.
Got that wrong on the real mcat
Besides, just memorizing the VSEPR chart of shapes and angles, does anyone have a useful or intuitive way of remembering all this information?
I recommend playing with Kinex, or a molecular modeling kit (though that's less fun when you're done).
Just open an old Chemistry Textbook and take a look down the entire list if you are really that worried. One readthrough and you will always remember it.
Just open an old Chemistry Textbook and take a look down the entire list if you are really that worried. One readthrough and you will always remember it.
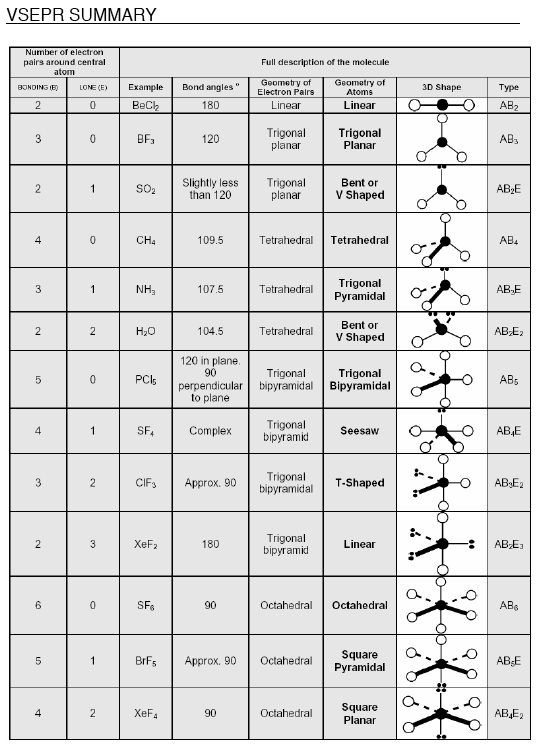
The only two angles I didn't already know here were the lone pair variants of tetrahedral.You're a badass if you can memorize that in one read through.
I'm here to prove you wrong.
You're a badass if you can memorize that in one read through.

Lone pairs repel, bond angles are as far apart as possible....
Maybe I just have a good visual memory?
Yea the names were always the hardest part for me. Especially because a lot of them have multiple names.

