The standard calculation method from general chemistry is cumbersome and will take several minutes (I will represent it second). But this question can be determined quickly and easily using PoE and intuition.
PoE and Intuition Method:
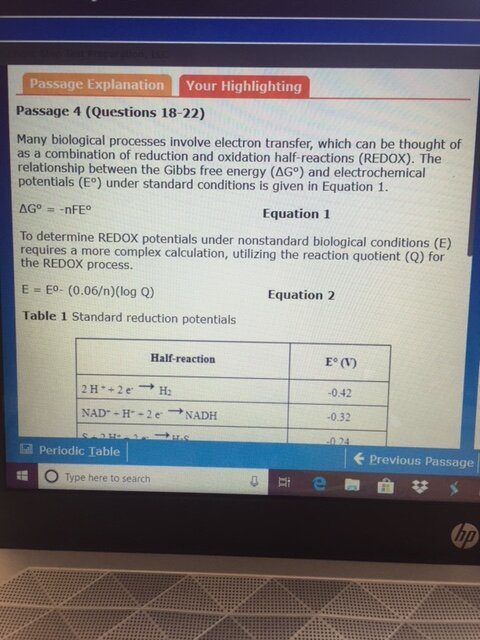
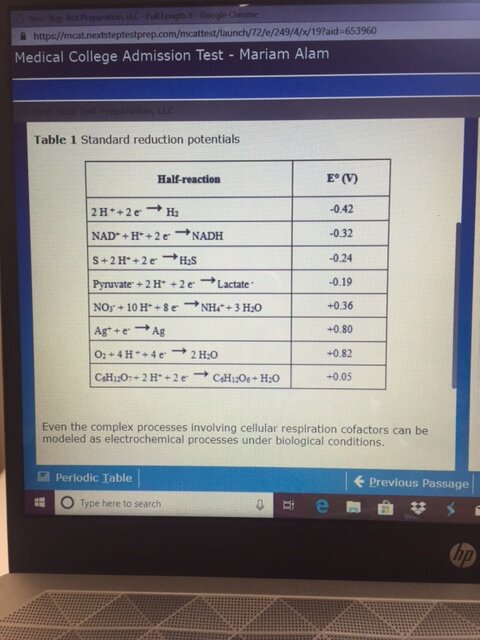
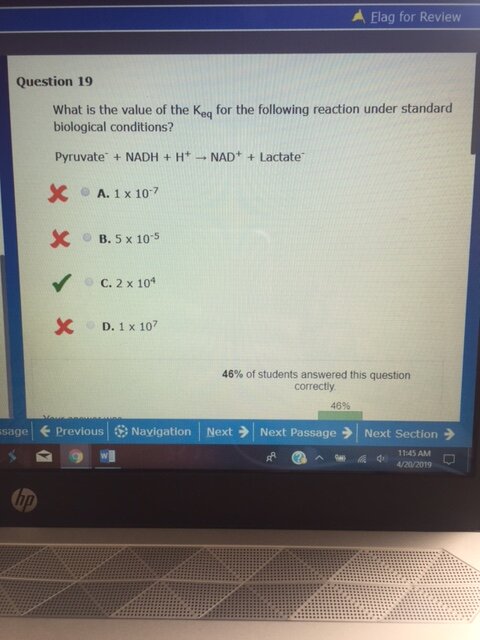
First, look at the half reaction table. NADH must be reversed, resulting +0.32 V. Pyruvate remains as is, making it -0.19. The overall redox reaction has E = +0.13. A positive E means that the reaction is favorable as written, so Keq is greater than 1. This eliminates choices A and B.
The easiest way to decide between C and D involves looking at the 0.06/n and 0.13 ratio. The value of n is 2 in this case (the number of electrons exchanged in the reaction), so we have 0.03 and 0.13 in the calculation. 0.03 does not divide into 0.13 evenly, so there is a remainder, which means that the first part of the answer cannot be 1.00. This eliminates choice D and leaves choice C.
Traditional Math-Based Method:
If you want a more precise answer, then we can use the Nernst Equation.
Consider that at equilibrium, deltaG = 0, which means that E is also 0. This is also where K = Q, so we can substitute K into the equation for Q and 0 for E.
0 = 0.13 - (0.06/2)logK = 0.13 - 0.03logK
0.03logK = 0.13
so logK = 0.13/0.03 = 4.33
This means that K = 10^4.33 = 10^0.33 x 10^4.
The log of 2 = 0.30, so 10^0.33 = 2.
This gives us 2 x 10^4.
In all honesty, I think the Math-based solution is far above and beyond the MCAT and would highly recommend the PoE method presented first. I'm not sure why AAMC would put this question on their practice exam, as this calculation seems beyond what they typically do.