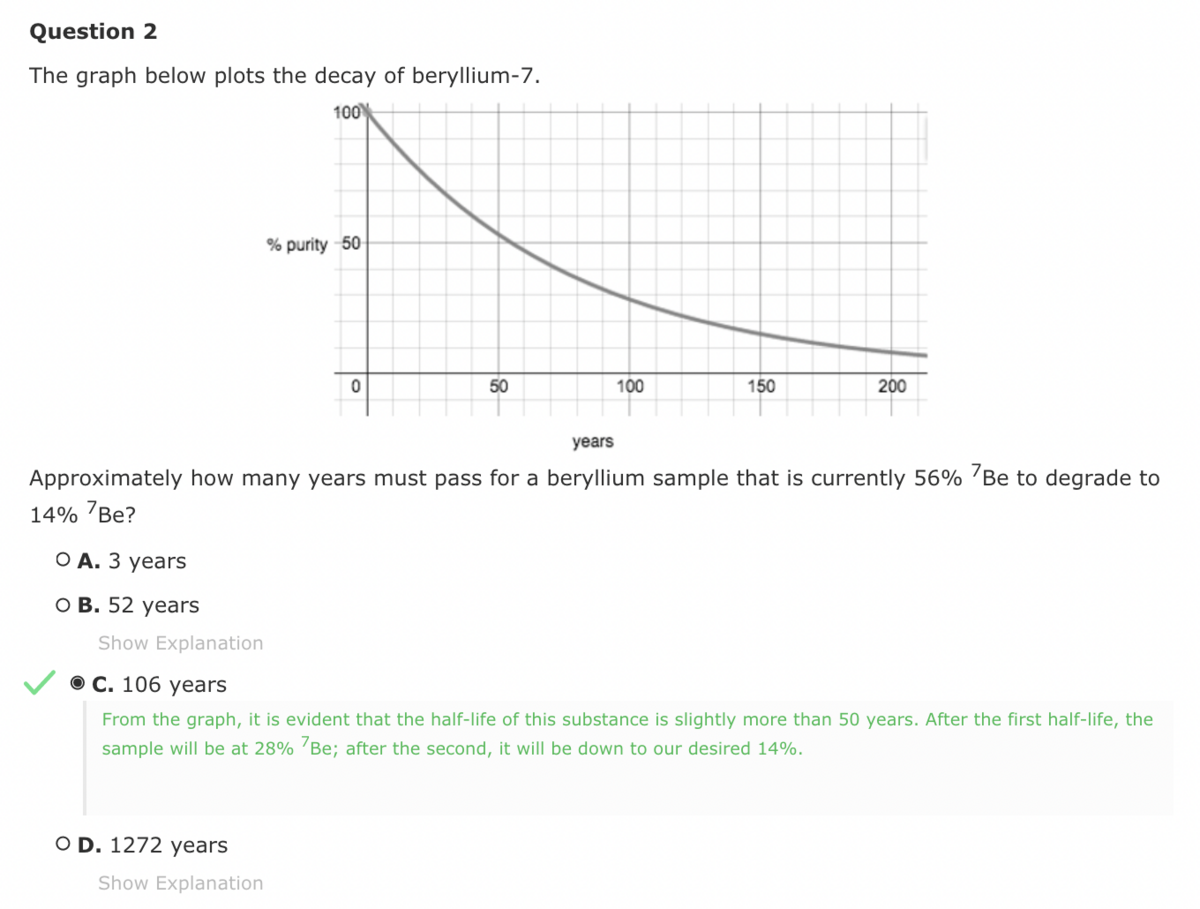
This is a first order plot, where the half life is constant. If you look at the graph, 100% is at t=0, 50% is around t=53, 25% is around t=106, etc...
So the easy way to set this up is to say that 56 —> 28 —> 14, meaning it is cut in half TWICE. That requires two half lives, which is 2 x 53 = 106.
I hope this helps.