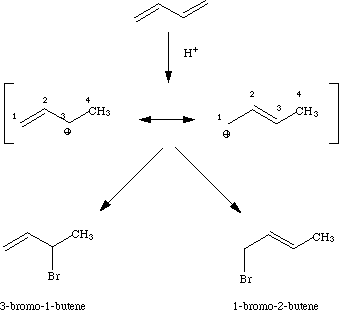
Sorry, upon further examination, I realize that mine needed a picture, so here it is:
As you can see, from the above, at 80 degrees C 3-Bromo 1-Butene is created faster, but at 40 degrees C, 1-Bromo-2Butene is created. When there is sufficient energy in the system, the system will go to create the fastest product, not the most thermodynamically stable product(It is better to have the double bond within the molecule than on a primary carbon.
Foghorn is 101.738% correct on this one. The author of your example screwed up their numbering of the intermediate and have the explanation completely backwards. I'd like to elaborate a bit.
A good way think about kinetic control versus thermodynamic control is to think of it in terms of economics. You have money to invest (similar to thermal energy floating around a system that can be used to overcome the activation barrier) and a desired return on your investment (energy released following reaction.)
If you have a
low temperature, then there is very little energy to invest. You are forced to choose the smallest investment, which is the one with the lowest activation energy. Because activation energy dictates the rate of a reaction, this pathway is refered to as the
kinetic pathway. Hence, as Foghorn has pointed out, the
lower temperature leads to the kinetic product.
If you have a
high temperature, then there is a large amount of energy to invest. You can choose either investment, so it is most probable that you will choose the one with the greatest return (most exergonic). Because heat/free energy dictates the favorablility of a reaction, this pathway is refered to as the
thermodynamic pathway. Hence, as Foghorn has pointed out, the
higher temperature leads to the thermodynamic product.
The conjuagated diene and the addition to the alpha-carbon of a carbonyl are classic examples of this.
{{{{{Opinionated sidenote}}}}} Whatever source you are using, you should be very careful, because this is a fundamental error that anyone on the caliber of material author should not make or at the very least catch upon a cursory editting. There are materials out there, some of them quite popular, that I find myself correcting many of their errors for during my open office hours. {{{{{end of Opinionated sidenote}}}}}