- Joined
- Jul 10, 2017
- Messages
- 46
- Reaction score
- 23
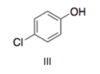
According to Mikes notes, the Halogens are weak Ortho Para directors, which means they are electron donors. So why is it when we are determining acidity these halogens are considered withdrawers? I thought this Cl would act as a donor, and decrease the acidity? How do we know when to consider this halogen as electronegative? or as a donor according the the O/P chart?