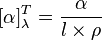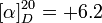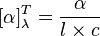For pure liquids
This equation is used:
In this equation, α (Greek letter "alpha") is the measured rotation in degrees,
l is the path length in decimeters, and
ρ (Greek letter "rho") is the density of the liquid in g/mL, for a sample at a temperature
T (given in degrees Celsius) and wavelength
λ (in nanometers). If the wavelength of the light used is 589
nanometers (
the sodium D line), the symbol “D” is used. The sign of the rotation (+ or −) is always given.
For solutions
A different equation is used:
In this equation, α (Greek letter "alpha") is the measured rotation in degrees,
l is the path length in decimeters and
c is the concentration in g/mL, for a sample at a temperature
T (given in degrees Celsius) and wavelength
λ (in nanometers).
[1] If the wavelength of the light used is 589
nanometer (the sodium D line), the symbol “D” is used. The sign of the rotation (+ or −) is always given. When using this equation, the concentration and the solvent may be provided in parentheses after the rotation. The rotation is reported using degrees, and no units of concentration are given (it is assumed to be g/100mL).
For example: