- Joined
- Sep 8, 2016
- Messages
- 124
- Reaction score
- 25
Hello SDN ,
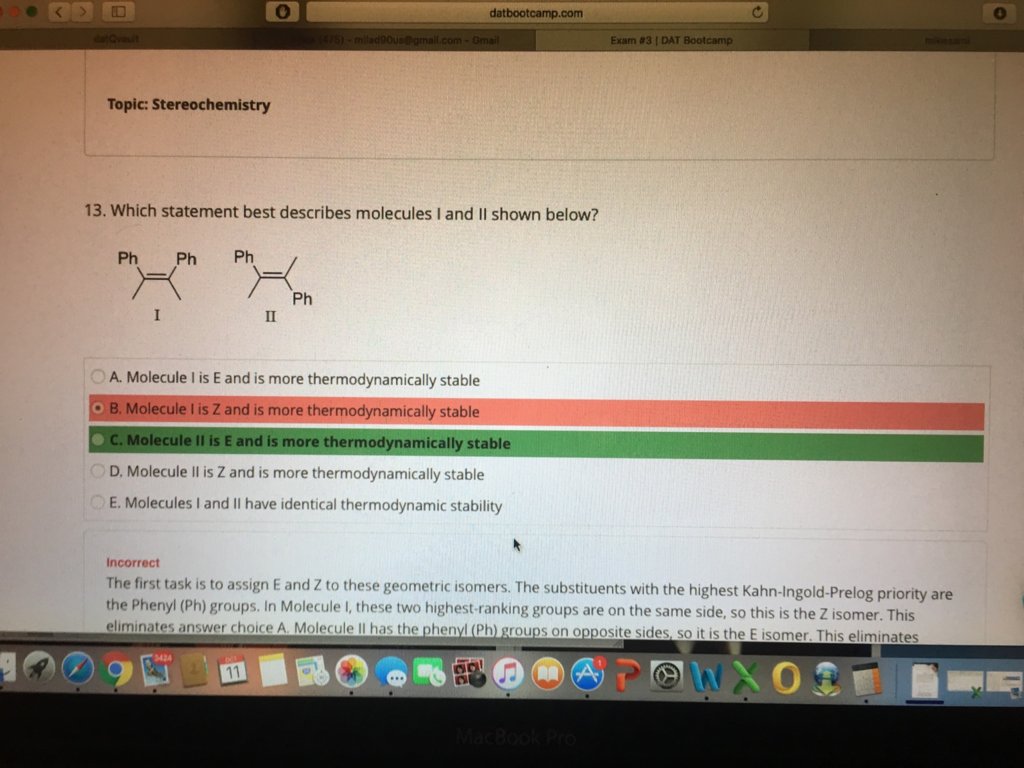
I was asking DR Romano about this question and I still got it wrong ! In my thinking process I though that E or Z the E will be non polar so it would be less thermodynamically favored! Am I confusing subject with CIS or Trans! I would love to get feedback from Dr, Romano on this!
Thanks!
Sent from my iPhone using SDN mobile
I was asking DR Romano about this question and I still got it wrong ! In my thinking process I though that E or Z the E will be non polar so it would be less thermodynamically favored! Am I confusing subject with CIS or Trans! I would love to get feedback from Dr, Romano on this!
Thanks!
Sent from my iPhone using SDN mobile

 thank you Dr Romano
thank you Dr Romano