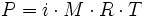think of it this way...
area where there is "less" water due to ions or whatnot water will attempt to flow into the area of lesser conc, same as diffusion.
So if you have an imperimable membrane and have ions present on one side water will be at a lower conc at that side due to the presence of the ions, so water from the side with no ions will flux to the side with ions.
Actually water flows both ways but the observation is a net increase or flux to the side with more ions, thats the bases of osmotic pressure.
,
where
i is the
van 't Hoff factor M is the
molarity R is the
gas constant, where
R = 0.08206 L · atm · mol-1 · K-1
T is the
thermodynamic temperature (formerly called absolute temperature)
van 't Hoff factor i is the number of moles of
solute actually in
solution per mole of solid solute added. Equivalently,
i refers to the ratio of true molecular mass to calculated molecular methods by
colligative methods
(thats from wiki)
be careful with the van't hoff factor something like 1 mole of NaCl will actually yeild TWO moles of ions (Na+ and Cl-)
so now if you have the typical exp with a tube bent upwards and water on each side with a membrane if you add salt to one side you will notice that the water level rises on that side, the difference between water levels on each side is the observed osmotic pressure due to water going from the hypotonic side (water with no ions) fluxing to the hypertonic side (water with ions) thus it attempts to balance the imbalance of water conc....