- Joined
- Jul 18, 2008
- Messages
- 6,239
- Reaction score
- 1,658
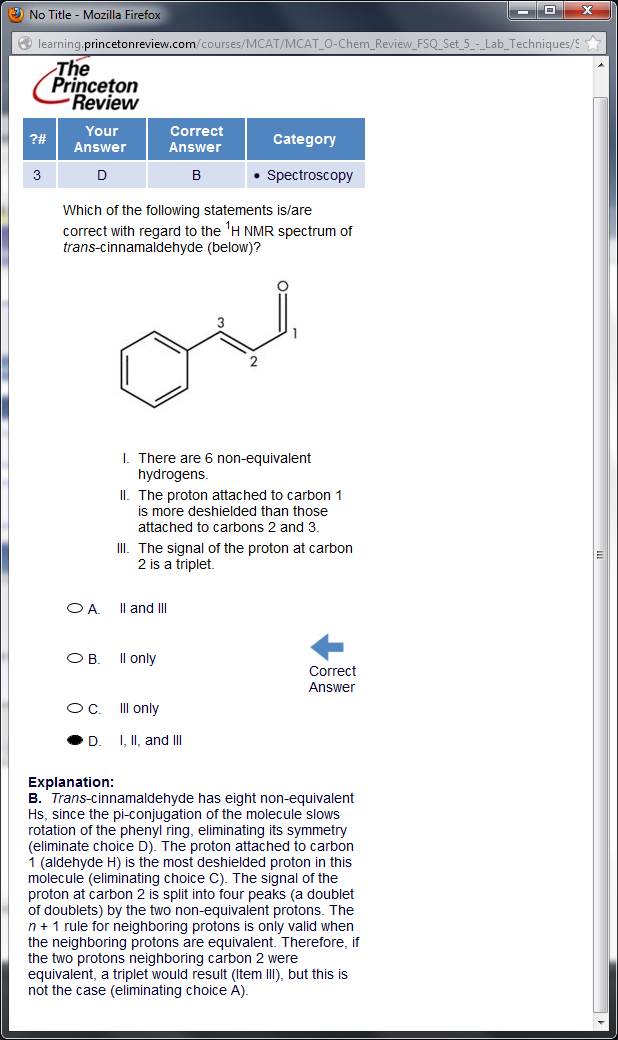
First of all

Second of all, is this something we should know for the MCAT? I've literally never heard of pi conjugation negating the equivalence of protons in an aromatic ring. Are there any other obscure "exceptions" that I should know about? I didn't know the MCAT tested on complex splitting, either.
