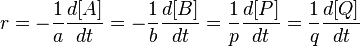TBR 9.4:
What is the reaction order for the following one step reaction:
2 NO2 -> 2NO + O2
So obviously it's going to be rate = k[NO]^2 is second order.
But what about the rate itself? Would it be, -1/2[NO]^2 = (1/2)[NO^2] = [O]?
What is the reaction order for the following one step reaction:
2 NO2 -> 2NO + O2
So obviously it's going to be rate = k[NO]^2 is second order.
But what about the rate itself? Would it be, -1/2[NO]^2 = (1/2)[NO^2] = [O]?