- Joined
- Feb 7, 2010
- Messages
- 152
- Reaction score
- 5
Here's a question I got right on TBR, but I think my understanding is still a little shaky despite having read the answer explanation.

So this is a graph of endothermic enthalpy, where the dH of the products is greater than that of the reactants.
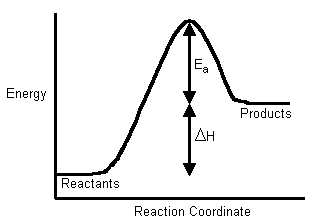
The answer explanation says that for an endothermic reaction the dG of the bonds broken are greater than then dG of the bonds formed. To break bonds you need to supply E in the form of H, hence why in this case the reaction is Endothermic because the products are at a higher E than the Reactants.
Lattice E is what holds the salts together and those are the bonds that are being broken,
My question where does the Solvation E come from? the H2O that's breaking the bonds?
And what bonds are being formed here if this salt is dissociating? Is it the individual ions (NH4+ and NO3-) being solvated by H2O mlcls?
In the future, how should I think about this problem? My logic was that if dG of the bonds being broken > dG of the bonds formed, then the products are going to have more dH due to the E released from the broken bonds? Am I thinking of this the right way?
So this is a graph of endothermic enthalpy, where the dH of the products is greater than that of the reactants.
The answer explanation says that for an endothermic reaction the dG of the bonds broken are greater than then dG of the bonds formed. To break bonds you need to supply E in the form of H, hence why in this case the reaction is Endothermic because the products are at a higher E than the Reactants.
Lattice E is what holds the salts together and those are the bonds that are being broken,
My question where does the Solvation E come from? the H2O that's breaking the bonds?
And what bonds are being formed here if this salt is dissociating? Is it the individual ions (NH4+ and NO3-) being solvated by H2O mlcls?
In the future, how should I think about this problem? My logic was that if dG of the bonds being broken > dG of the bonds formed, then the products are going to have more dH due to the E released from the broken bonds? Am I thinking of this the right way?
