- Joined
- May 10, 2015
- Messages
- 708
- Reaction score
- 614
1) Given this picture: what is the most acidic proton?
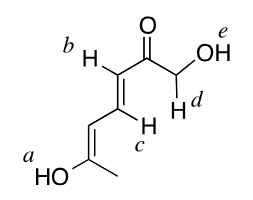
Proton e has inductive stabilization from the carbonyl but proton A has resonance stabilization from the conjugated system. What is more important when determining acidity: inductive stabilization, or resonance? Why?
2) Which of the following 5 structures has the most stable resonance form?
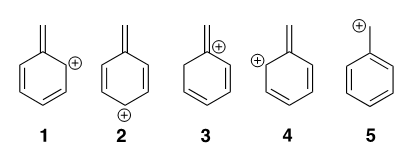
First, is the question equivalent to: which is the most stable carbocation? If so, how does having a benzene ring attached to a carbocation stabilize it? I understand that structures 1, 2, 4, and 5 are all allylic carbocations but don't necessarily understand why 5 would be more stable than 1, 2 and 4.
3) relationship of physical and chemical properties between stereoisomers:
- enantiomers have identical and physical properties except for their ability to rotate plane polarized light?
- cis/trans isomers have different physical and chemical properties?
- how do the physical and chemical properties of 2 diastereomers relate?
thanks
Proton e has inductive stabilization from the carbonyl but proton A has resonance stabilization from the conjugated system. What is more important when determining acidity: inductive stabilization, or resonance? Why?
2) Which of the following 5 structures has the most stable resonance form?
First, is the question equivalent to: which is the most stable carbocation? If so, how does having a benzene ring attached to a carbocation stabilize it? I understand that structures 1, 2, 4, and 5 are all allylic carbocations but don't necessarily understand why 5 would be more stable than 1, 2 and 4.
3) relationship of physical and chemical properties between stereoisomers:
- enantiomers have identical and physical properties except for their ability to rotate plane polarized light?
- cis/trans isomers have different physical and chemical properties?
- how do the physical and chemical properties of 2 diastereomers relate?
thanks

